
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
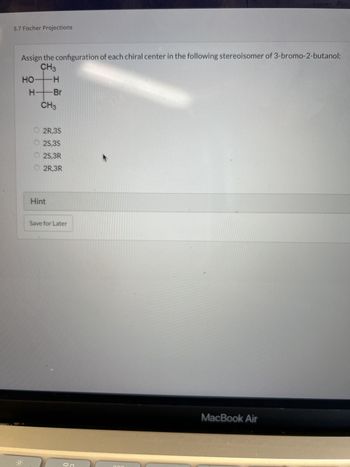
Transcribed Image Text:**Title: 5.7 Fischer Projections**
**Question:**
Assign the configuration of each chiral center in the following stereoisomer of 3-bromo-2-butanol:
**Diagram:**
- A Fischer projection showing a central carbon chain.
- The vertical line represents the carbon chain with the structure:
- Top Carbon (1): CH₃ group
- Second Carbon (2): HO on the left, H on the right
- Third Carbon (3): H on the left, Br on the right
- Bottom Carbon (4): CH₃ group
**Answer Options:**
- ⃝ 2R, 3S
- ⃝ 2S, 3S
- ⃝ 2S, 3R
- ⃝ 2R, 3R
**Controls:**
- Hint button
- Save for Later button
**Description:**
This exercise requires the determination of the stereochemical configuration (R/S) for each chiral center in the Fischer projection of 3-bromo-2-butanol. The options provided indicate possible configurations for the chiral centers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is a structural formula for cortisol (hydrocortisone). Draw a stereo-representation of this molecule showing the conformations of the five- and six-membered rings.arrow_forwardHow many chiral center(s) are present in each molecule and how many stereoisomers are possible in each case? a) 2- Chloropentane b) 3- chloropentane c) 3- Chloro- 1- pentene draw the structures of each of the molecules on paper with showing chiral centers with a star/asterisk and mention the number of the stereoisomers for each molecule.arrow_forwarda) How many chiral centers exist in the following molecule structure A? b) How many possible stereoisomers would exist for structure A (including structure A) based on the correct number of chiral centersarrow_forward
- Answer all questions pleasearrow_forwardHelp me with my study guidearrow_forward8. The Fischer projection formula for D-mannose, a hexose sugar, is shown below. How many chiral centers are in D-mannose, please circle each Chiral C? HO- HO- H H CHO -H H -OH -OH CH₂OH Number of Chiral Centers and circle each in the structure: Study Guide Practice Questions 9. Draw the structure for 2-chloro-4-methylhexanal 10. What is the IUPAC name of the compound shown below? CH3 CH₂-C CH₂CH₂CH₂CHCH₂arrow_forward
- Hi can you please please help me with these questions thank you so mucharrow_forwardAll the following questions have to do with a molecule with the formula of C10H11NO3 a) Draw the BOND LINE structure of an ACHIRAL molecule with no CHIRAL CENTERS b) Draw the BOND LINE structure of a CHIRAL c) Draw the BOND LINE structure for the ENANTIOMER in question b d) Draw the BOND LINE structure for the MESO compound e) Draw the BOND LINE structure for the moleculearrow_forward2-hydroxypropanoic acid (lactic acid) has a chiral carbon atom. Draw the structure of lactic acid and mark the chiral atom with *. Draw three-dimensional diagrams of the two optical isomers to show that they are non-superimposable mirror images of each other. 1. Locate the chiral centre 2. Draw one enantiomer 3. Draw its mirror image beside it. in a tetrahedral shape - Draw and name the geometric isomers of C2H2CI2 3. Draw its mirror image beside it. 2. Draw one enantiomer in a tetrahedral shape 1. Locate the chiral centrearrow_forward
- 15. Naming Stereoisomers with Two Chiral Carbons Using the RS System The (RR) isomer of methyphenidate (Ritalin) is used to treat attention deficit hyperactivity disorder (ADHD). The (S.S) isomer is an antidepressant. Identify the two chiral carbons in the structure below. Is this the (R.R) or the (S.S) isomer? Draw the other isomer. HN- H. ..arrow_forwardUsing only C, H and O, write a structural formula for the lowest-molecular-weight chiral molecule of each of the following compounds a) Alkane b)Alcohol c)Aldehyde d)Ketone e)Carboxylic Acid I realise this question has been asked but the previous seen formulas do not work for my homework.arrow_forwardWe don't see the answer written with a photo or pen, give the answer using the toolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning