
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Dd.50.
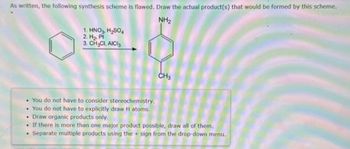
Transcribed Image Text:As written, the following synthesis scheme is flawed. Draw the actual product(s) that would be formed by this scheme.
NH₂
1. HNO3, H₂SO4
2. H₂, Pt
3. CH₂CI, AICI
CH3
• You do not have to consider stereochemistry.
. You do not have to explicitly draw H atoms.
• Draw organic products only.
If there is more than one major product possible, draw all of them.
. Separate multiple products using the sign from the drop-down menu.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the blanks for 1-6arrow_forwardalculate the approximate volume of the vinegar sample needed for the analyses (Part A.1). Brand of vinegar or unknown no. Colauita tof White Wine Trial 1 1122809 Trial 2 Trial 1 Trial 2 e Guia 112.280g (12.822g 112.796 115 565 lis.9 1. Mass of flask (g) l15.603 2. Mass of flask + vinegar (g) 2.496 3.323 2,743 2,749 3. Mass of vinegar (g) B. Analysis of Vinegar Sample 2mL 7.5 23mL 32.コ 1. Buret reading of NaOH, initial (mL) 5.25mL 13ML amL 2. Buret reading of NaOH, final (mL) 33,3 31.2 1. Volume of NaOH used (mL) 2! mL a7.45 me 20.3 32.2 o i135 • 004 1 Molar concentration of NaOH (mol/L) Oi135 • 00 3 1003 , 063 5. Moles of NaOH added (mol) 6. Moles of CH,COOH in vinegar (mol) 7. Mass of CH,COOH in vinegar (g) 8. Percent by mass of CH,COOH in vinegar (%) 9. Average percent by mass of CH,COOH in vinegar (%) Calculations for Trial 1 of the first vinegar sample on next page. Experiment 10 137arrow_forward1:39 1 Send a chat Enter your answer in the provided box. Hydrogen fluoride is used in the manufacture of Freons (which destroy ozone in the stratosphere) and in the production of aluminum metal. It is prepared by the reaction CaF2 + H2SO4 → CASO4 + 2HF In one process, 5.75 kg of CaF, is treated with an excess of H,SO, and yields 2.45 kg of HF. Calculate the percent yield of HF. % yieldarrow_forward
- The ______ is a mixture of gases: mostly nitrogen and oxygen, with smaller amounts of argon, carbon dioxide, and other gases.arrow_forward2. Calculate the following, with proper number of significant figures: Molar mass of Al(CH3COO)3 is g/mol In 16.4 moles of Al(CH3COO)3 ... ... the number of grams of Al(CH3COO)3 is the count of AlL(CH;COO); units is ... the number of moles of 6C atoms is mol ... ... the count of 6C atoms is ... the mass of 6C atoms is g The % mass fraction of 6C atoms in Al(CH;COO); is % The % mass fraction of Al atoms in Al(CH;coo)3 is % Name the compound:arrow_forward87.) All the fertilizers listed below contribute nitrogen to the soil. If all these fertilizers are sold for the same price per gram of nitrogen, which will cost the least per 50-lb bag? Urea, (NH2)2CO Ammonia, NH3 Ammonia nitrate, NH4NO3 Guanidine HNC(NH2)2arrow_forward
- Combustion of a 0.9827-g sample of a compound containing only carbon, hydrogen, and oxygen produced 0.5186 g of C and 0.1197 g of H. What is the empirical formula of the compound? For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph V Arial RBC P ¶ X 10pt 88 8 AC !!! 關 +88 A V तं {6} Ix XQ R7 Ky 5 |||| ווין |||| 三三 x²arrow_forward30 CI 'N ZI MeCN (Solvent) ?arrow_forwardIdentify one substrate and one reagent that could be combined to make the completed product.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY