
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:0507 CHE114 POGILS11_12 Google...
phrasing
Extensions Help
mal text
12 + BI UA
6
7
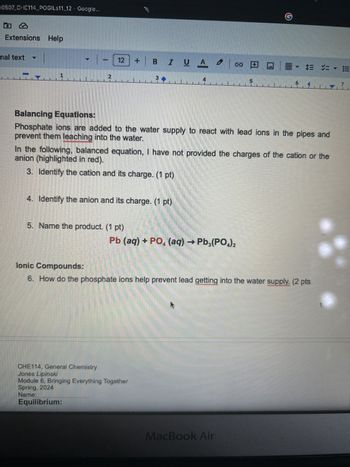
Balancing Equations:
prevent them leaching into the water.
Phosphate ions are added to the water supply to react with lead ions in the pipes and
In the following, balanced equation, I have not provided the charges of the cation or the
anion (highlighted in red).
3. Identify the cation and its charge. (1 pt)
4. Identify the anion and its charge. (1 pt)
5. Name the product. (1 pt)
Pb (aq) + PO4 (aq) → Pb3(PO4)2
Ionic Compounds:
6. How do the phosphate ions help prevent lead getting into the water supply. (2 pts
CHE114, General Chemistry
Jones Lipinski
Module 6, Bringing Everything Together
Spring, 2024
Name:
Equilibrium:
MacBook Air
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- ew ntent u.co sk... History X Favorites Tools Profiles Tab Window Aktiv Chemist X Microsoft Office H... 1 C Write a balanc X Blackboard + 04- 2 0₂ Ac C Pearson - Course... 3. 3 3 ) Help Q Determine the X Write a balanced chemical equation based on the following description: aqueous iridium(III) bromide reacts with aqueous silver acetate to produce solid silver bromide and aqueous iridium(III) acetate IrBr₂(aq) + AgC₂H₂O₂(aq) 4 0²- 0- 5 ->> 1 O Time's Up! Ox 05 0+ 11 6 ☐6 7 (s) ²+ 07 Ir MacBook Pro Grams to Mol X H Br 21 3+ 8 08 (1) CO zoom 04+ 0 9 Si Ag Determine the X A 0 (g) (aq) $ 口。 O 73% C Detern € 61arrow_forwardA chemist adds 215.0 mL of a 0.19M barium acetate solution to a reaction flask. Calculate the mass in grand of barium acetate the chemist has added to the flask. Round your answer to 2 sig figs.arrow_forwardB) Find the normality of a solution that can be made by dissolving 2.8 g. of CaCl, in 250 ml of water. (Atomic weights for Ca is 20 and for Cl is 35) C) A compound of iron and oxygen is analyzed and found to contain 69.94% iron and 30.06% oxygen. Find the empirical formula of the compound. (Where the atomic weight of O is 16 and for Fe is 55.85).arrow_forward
- Solve using dimensional analysis. Show the complete solution. Do not round off nonfinal answers. Box your final answer.arrow_forwardA 623 mL NaCl solution is diluted to a volume of 1.45 L and a concentration of 4.00 M. What was the initial concentration? Express your answer with the appropriate units. • View Available Hint(s) HA M1 = 8.88 M Submit Previous Answers X Incorrect; Try Again; 4 attempts remaining Review your calculations and make sure you round to 3 significant figures in the last step.arrow_forward个 app.101edu.co STARTING AMOUNT esc q X 2 #3 3 How many grams of AgCl will be formed when 60.0 ADD FACTOR x( ) completely reacted according to the balanced chemical reaction: FeCl3(aq) + 3 AgNO3(aq) 6.022 × 1023 Oll 4 4.30 0.001 Question 31 of 34 169.88 FS % 5 143.32 1 6 → 0.500 3 AgCl(s) + Fe(NO3)3(aq) y 3 g AgCl LAgNO3 LAgCl MAGNO, mol AgCl mL AgNO3 OM MOO mL of 0.500 M AgNO3 is ANSWER 7 7 1.43 1000 U 60.0 8 RESET 2 12.9 1.70 mL AgCl Mar 2 5:27 delete ★ ✰ ☐ Submit + 0 backspace 180arrow_forward
- Using molarity to find solute mass and solution volume g 3/5 A chemist adds 105.0 mL of a 0.721M barium chloride (BaCl₂) solution to a reaction flask. Calculate the mass in grams of barium chloride the chemist has added to the flask. Round your answer to 3 significant digits. Ś Uyen Kha "...arrow_forwardPage of 2 + ZOOM 1. Balance these equations. a. Al(s)+O2(g)¬→Al½O3(s) b. N2(g)+H2(g)→NH;(g) c. C,H6(1)+O2(g)→H2O(1)+CO2(g) 2. Balance the following equations. а. СаС2(8)+Н-О() --Са(ОН)2(s)+СH-(g) b. (NH4)2Cr2O7(s)→Cr2O3(s)+N2(g)+H2O(g) c. CH;NH2(g)+02(g)→CO2(g)+N2(g)+H2O(g) 3. An explosive whose chemical formula is C;H,N6O6 produces water, carbon dioxide, and nitrogen gas when detonated in oxygen. Write the chemical equation for the detonation reaction of this explosive. 4. A number of compounds are used in cement, and reactions among them occur when water is added. In one, CaO reacts with Al2O3 and water to form Ca3Al2(OH)12. Write a balanced chemical equation for this process. 5. Ethanol, C2H5OH, is found in gasoline blends used in many parts of North America. Write a balanced chemical equation for the combustion of C2H5OH to form CO2 and H2O. 6. Balance the following equations. a. reaction to produce "superphosphate" fertilizer: Ca3(PO4)2(s)+H2SO:(aq)→Ca(H,PO4)2(aq)+CaSO4(s) b.…arrow_forwardPart A. Chemical Reactions: Describe observations 1. Composition and Redox: 2 Mg (s) + O2 (g) → 2 MgO (s) Observations of the reaction 2. Decomposition and Redox: 2 Ag2O (S) → 4 Ag (S) + O2 (g) -> A. Initial observations of Ag20 (s) B. Observations when tube is heated 362 words D Focus Type here to searcharrow_forward
- Balance CaCL2(aq) + Na3PO4(aq) —> Ca3(PO4)2(s)+ NaCL(aq). Identify single displacement /combination/ decomposition/double displacementsarrow_forwardy D a -h 14. The unknown tablet is prepared for analysis in two steps. The tablet is crushed into a powder and then dissolved into a 250 mL volumetric flask with dilute acetic acid. A diluted powder solution is prepared by transferring 25.00 mL of the original powder solution into a 50 mL volumetric flask and diluting to volume with dilute acetic acid. Then 1.00 mL of the diluted powder solution is added to each of the standard addition flasks; each flask has a total volume of 10.00 mL. The unknown's riboflavin concentration in the standard addition solutions is determined to be 1.41 ppm. What is the riboflavin concentration (ppm) in the unknown powder solution? 2.82 21.2 14.1 28.2arrow_forward1 of 4 Watch the video that shows using the dilution method to make a solution of known concentration. Note that while the video shows the use of a beaker, in the lab we typically use a volumetric flask to make the diluted solution. The stock solution of KMNO, has a concentration of 2.6 x 10ª M. The pipette has a volume of 10.0 mL. What is the amount of KMNO. delivered to the solution, in moles? mol Submit When using the dilution method to prepare a solution of known concentration, remember that the total amount of solute is the same in both the measured volume of the more concentrated stock solution and in the dilute solution created. This means we can use a simple ennation (Emation 4 2) to calculate the volume or concentration of either the stock solution or the more dilute solution Fauation 4 2 is stv hulu Warrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY