
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
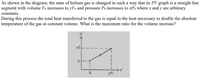
Transcribed Image Text:As shown in the diagram, the state of helium gas is changed in such a way that its PV graph is a straight line
segment with volume Vo increases to yVo and pressure Po increases to xPo where x and y are arbitrary
constants.
During this process the total heat transferred to the gas is equal to the heat necessary to double the absolute
temperature of the gas at constant volume. What is the maximum ratio for the volume increase?
xP,
P.
Vo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- In the air conditioning process in the humidifier, air at a dry bulb temperature of 30 degrees C and RH of 10% increases its RH to 50%. Determine the amount of moisture added in the humidifier per kg of dry air: ....arrow_forwardPLs help me answer this asaparrow_forwardIn the 1970s cartoon The Super Friends, the Wonder Twins helped Superman and others fight crime. One of the Wonder Twins was Zan, who was able to take on the form of H2O in its various phases (ice, liquid water, water vapor). After taking a physics class, he decides to conduct a little experiment by turning into an ice igloo in the park on a hot day. (a) If his total mass as an igloo is 64.50 kg and he starts with a temperature of −13.50°C, how much heat must flow into him to completely melt his body? J(b) He continues absorbing energy up to the point where he would start to vaporize (100.0°C). How much more heat is required to raise his temperature to the vaporization point? J(c) How much more heat is required to turn him into water vapor at 100.0°C? J(d) If we model the water vapor molecules making up his body as having only six degrees of freedom, what is the average kinetic energy of one of the water vapor molecules that make up Zan? (Consider the case when Zan is water vapor at…arrow_forward
- 70 Energy (Joule) ☎ 8 8 8 8 8 8 50 30 10 o S2FRT820 $2FRT730 AS2FRT650 S2HT940 o $2HT1150 0 -200 -150 S2FRT820 O -100 + S2HT940 A -50 0 Temperature (°C) O O S2FRT730 ... S2FRT650 $2HT1150 50 100 The figure shown corresponds to the absorbed energy of different ferritic steels in a temperature range of 1. What is the ductile-brittle transition temperature of S2FRT730 steel? 2. If a component will work at -50°C, what materials (from those shown) would you use to manufacture it? EXPLAIN YOUR ANSWER.arrow_forwardThe graph below shows the pressure and volume for a sample of dry air carried out at two different constant temperatures. Volume (x 10-³ m ³) 2.5 N 1.5 1 0.5 0 50 M 100 150 Pressure (k Pa) 200 250 -T= 300 K -T200 K An isothermal change has to be carried out very slowly. Explain why this is the case. Use data from the graph and the ideal gas equation to calculate the number of moles of gas in the sample.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY