
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
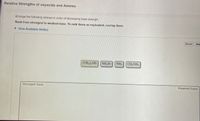
Transcribed Image Text:Relative Strengths of oxyacids and Amines
Arrange the following amines in order of decreasing base strength.
Rank from strongest to weakest base. To rank items as equivalent, overlap them.
> View Available Hint(s)
Reset
Hel
(CH3)2NH
NH,Br
NH3
CH3NH2
Strongest base
Weakest base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- from the three compounds on the left side, identify which one is most nucleophilic and which one is most basic.arrow_forwardWhich compound is least basic ? Explain. -NH₂ tâNH CHO fan CHO NH O CHE WH₂arrow_forwardBr ||| Based on resonance and inductive effects, identify the strongest base among the aniline derivatives shown below. NH₂ NH₂ O₂N7 IV H3C NH₂ Question 30 of 30 NH₂ A) I B) II C) III D) IVarrow_forward
- Hello, can you please help me figure out why these are wrong, I am using them to study and am very confused. Draw the products formed when each of the amides is treated with water and HCl. Hint: Consider the structure of carboxylic acids and amine salts. Remember to include the appropriate charge on the amine salt and to include the Cl− ion.arrow_forwardOnly typed explanation otherwise leave itarrow_forwardCan someone please explain the logic behind the ranking?arrow_forward
- Rank and EXPLAIN WHY pleasearrow_forward-NH2 ج جب ON NH2 Rank the following structures in order of increasing basicity. H3CO III NH2 A) | < II < ||| B) II < I < ||| C) III < I < || D) I < III < || E) ||| < || < |arrow_forwardRank the following organic molecules in order of decreasing acidity. That is, select '1' in the second column for the most acidic molecule, '2' for the next most acidic molecule, and so on. HO HO Jl. CI OH OH (Choose one) (Choose one) (Choose one) ▼ (Choose one) ▼ Xarrow_forward
- Give detailed Solutionarrow_forward(b) Identify the most acidic H on each molecule, then list its approximate pka in the box provided. H2N Но Но H3Narrow_forwardFor the following acid-base reaction, predict which side of the equilibrium is favored. O Right-hand side O Left-hand side O Both are alkane protons, so neither side is favored.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY