
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
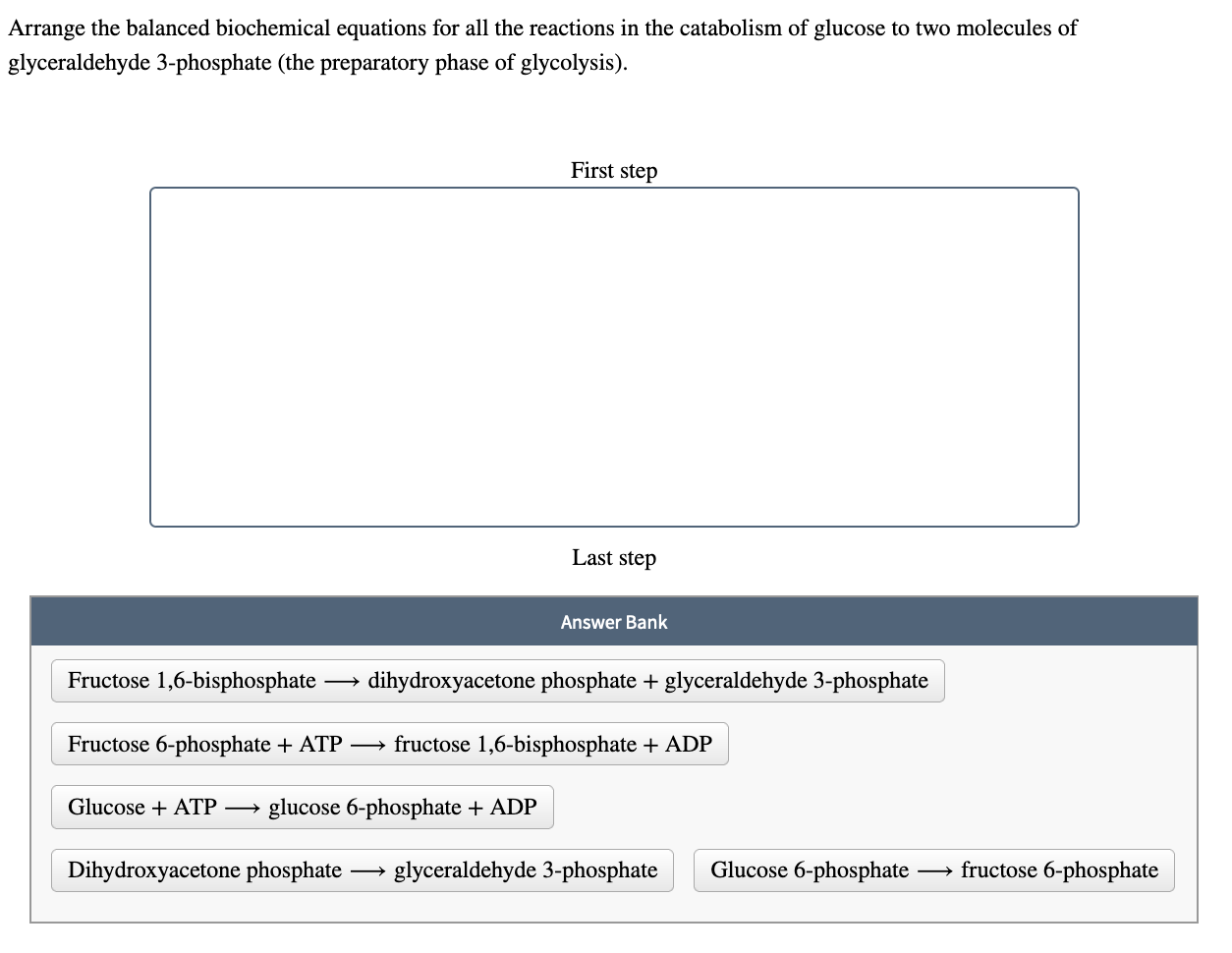
Transcribed Image Text:Arrange the balanced biochemical equations for all the reactions in the catabolism of glucose to two molecules of
glyceraldehyde 3-phosphate (the preparatory phase of glycolysis).
First step
Last step
Answer Bank
Fructose 1,6-bisphosphate - dihydroxyacetone phosphate + glyceraldehyde 3-phosphate
Fructose 6-phosphate + ATP →→→→ fructose 1,6-bisphosphate + ADP
Glucose + ATP → glucose 6-phosphate + ADP
Dihydroxyacetone phosphate glyceraldehyde 3-phosphate Glucose 6-phosphate →→→→ fructose 6-phosphate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 5arrow_forwardWhich of the following accurately characterize glycolysis under standard biochemical conditions? (Pick two.) Glycolysis reduces glucose and produces oxidized electron carriers. Glycolysis oxidizes glucose and produces reduced electron carriers. The exergonic conversion of glucose into pyruvate is coupled to the endergonic synthesis of two ATP in a pathway that is exergonic, net. The endergonic conversion of glucose into pyruvate is coupled to the endergonic synthesis of two ATP in a pathway that is exergonic, net.arrow_forwardSaccharides: Using the following substrates, estimate the net ATP yield after glycolytic pathway, Kreb’s cycle and electron transport chain. Assume that the estimate for ATP yield per mole of NADH is 3 moles of ATP, while 1 mole of FADH2 is equivalent to 2 moles of ATP, and one mole of GTP is equivalent to one mole of ATP. Show all pertinent solutions and determine: a) ATP used, b) ATP produced, and c) Net ATP. Based on your solutions, rank the substrates based on increasing yield of ATP Two moles of glyceraldehyde-3-phosphatearrow_forward
- Please help fill out the metabolic Pathway table of Gluconeogenesis and Tricarboxylic by listing its enzymes and functionsarrow_forwardIndicate what will happen (increase, decrease or no effect) to the activity of the enzyme or rate of the metabolic pathway given the following conditions.arrow_forwardHexokinase phosphohexose isomerase [Choose ] [Choose ] Is not the trapping reaction catalyzes an ATP-dependent phosphorylation of its substrate. Is irreversible and carries out substrate-level phosphorylation. PFK-1 aldolase triose-phosphate isomerase Splits the carbohydrate in two. Product is 2-phosphoglycerate. Is reversible and carries out substrate-level phosphorylation. Product is fructose-6-phosphate. Catalyzes the trapping reaction Makes a 2nd mole of G3P from DHAP Produces a high-free energy compound but no NADH or ATP. Oxidizes an aldehyde. GAPDH [Choose ] Phosphoglycerate kinase [Choose ]arrow_forward
- In class, I mentioned that fructose is metabolized differently in the liver compared to glucose. Refer to the figure shown below to calculate the number ofATPs you would expect from the metabolism of fructose in the liver. Show your work! Fructokinase Fructose Fructose-1-P АТР ADP Aldolase B Dihydroxy- acetone phosphate Glyceraldehyde АТР Triose kinase Triose phosphate isomerase ADP 4 - Glyceraldehyde-3-P Glycolysis Руruvate Acetyl-CoA Fatty acids and triglyceridesarrow_forwardOrder the cofactors based on their use in the mechanism of the a-etogluterate dehydrogenase complex. 1. Stabilizes a carbanion due to decarboxylation 2. Allows for the splitting of the carbon skeleton from the electron pair generated in a redox reaction 3. Enzyme bond electron carrier that is part of dihydrolipoyl dehydrogenase 4. Final electron acceptor in the overall reaction catalyzed by this complex 1 2 3 4 answer choices: lipoamide, biotin, 2 Fe - 2S cluster, TPP, NAD+, FADarrow_forwardPlease select the correct oparrow_forward
- Allosteric regulation plays a very important role in the regulation of glycogen metabolism. Which of thefollowing is correct? -AMP allosterically inhibits glycogen phosphorylase -Glucose-6-phosphate allosterically activates glycogen synthase -Both A and B -Neither A nor B The value of standard free energy change for reaction A --> B is +3.7 kJ/mol. Under which of thefollowing conditions, the reaction will most likely become exergonic in the direction as written? -Mass action ratio is larger than the equilibrium constant -Couple with ATP hydrolysis -Both A and B -Neither A nor Barrow_forwardIn different organisms sucrose can be cleaved either by hydrolysis or by phosphorolysis. Calculate the ATP yield per mole of sucrose metabolized by anaerobic glycolysis starting with (a) hydrolytic cleavage and (b) phosphorolytic cleavage.arrow_forwardCalculate the energy(Kcal.) produced from full oxidation of (3) mole of glucose (glucose --> CO2+ H2O), Compare the energy yield with that produced from oxidation of (1) mole .of stearic acid (C18)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON