
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
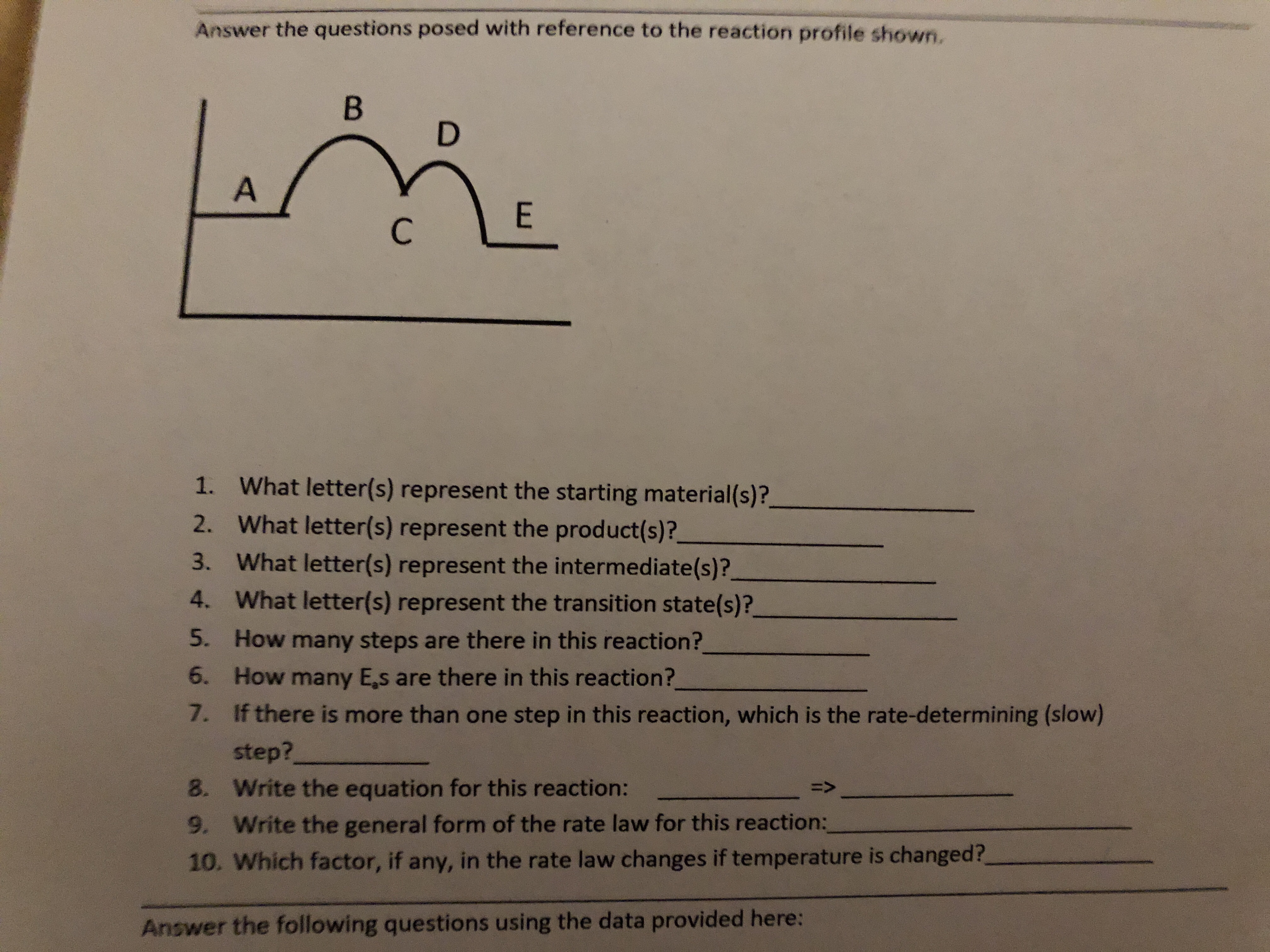
Transcribed Image Text:Answer the questions posed with reference to the reaction profile shown.
В
D
A
E
С
1.
What letter(s) represent the starting material(s)?
2.
What letter(s) represent the product(s)?
3.
What letter(s) represent the intermediate(s)?
4.
What letter(s) represent the transition state(s)?
5.
How many steps are there in this reaction?
6.
How many Es are there in this reaction?
7.
If there is more than one step in this reaction, which is the rate-determining (slow)
step?
8.
Write the equation for this reaction:
9. Write the general form of the rate law for this reaction:
10. Which factor, if any, in the rate law changes if temperature is changed?
Answer the following questions using the data provided here:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- Determine the reactants and reaction conditions in the correct order to complete the next conversion.arrow_forward4. Consider the following reaction mechanism and answer the questions. H-Br CH2 -CH3 -CH3 -CH3 Br Br Product B Br, Br Product A -CH3 a) Which product is most stable, label the kinetic and thermodynamic products? b) Which would have a higher energy of activation for the step leading to the kinetic or thermodynamic product (second step)? c) Sketch a reaction coordinate representing the above mechanism assuming the first step is the rate determining step. Include both pathways, label reactant, products, energy of activation and energy of reaction for the thermodynamic product. Reaction Coordinate Free Energyarrow_forwardProducts Reactants Extent of reaction Which letter shows the catalyized reaction? B. both DOOarrow_forward
- Complete the following reaction schemes by choosing the correct product, starting material, and/or reagent from the selection below. Your answer should simply be the number that represents your choice. For example, if you believe that the correct answer is the structure represented by the 3, then enter 3 into the correct answer box. Also, answer any additional questions that might be included with a reaction scheme(s). H₂C 0 1. NaNH, 1. NaNH, 2. CH₂CH₂CH₂Br 2. CH₂CH₂Br OH 1 4 3 2 5 O 6 Naº NH3 (1) 11 OH 7 7 XXXXXXXXX E RUTINE H H₂ Pd/C, CaCO3 8 1. NaNHz 2. CH₂(CH₂)₂CH₂Br 13 MCPBA 12 A B a. What reagent(s) is/are required for reaction A to proceed as drawn? What reagent(s) is/are required for reaction B to proceed as drawn? H₂C 9 H₂ Pd/C 14 -0 CH3 Br 10 1. BH-THF 2. H,O2, HỌ, H,O 15arrow_forwardWhich species does the first transition state of the overall reaction most ressemble? What kind of transition state is this (late or early)?arrow_forwardin the reaction above, how would the reaction shift (left or right) if we added extra NO2 to the reaction? What would the reaction color be after the shift?arrow_forward
- What is the complete mechanism of this reaction?arrow_forwardRemaining Time: 1 hour, 16 minutes, 57 seconds. v Question Completion Status: A Moving to another question will save this response. Question 22 Arrange the following compounds in order of increasing reaction rate with HNOg04 2 1 O 3<1<2<4 O 3<4<1<2 O1<3<4<2 O1<2<3<4 A Moving to another question will save this responsearrow_forward5. Draw the energy diagram for the following reaction in the reaction coordinate drawn below. HCI + H20 –→ H3O+ + Cl In your energy diagram, account for the fact that a) the reaction is very fast b) the reaction is strongly exergonic Clearly label all reactants, products, transition states, intermediates, and their energies.arrow_forward
- Consider the following reaction coordinate diagram. Energy (kJ) 50 45 40 30 20 15 10 5 0 Extent of reaction (a) Which is the rate-limiting step in this reaction?arrow_forwardAn equilibrium-controlled reaction will yield: Select one: ⒸA. the product that forms the fastest. ⒸB. the product whose formation involves the smallest energy of activation. OC. the product that can be formed in the fewest steps. OD. the most stable product.arrow_forward7. The given reaction is set to proceed via which mechanism? (c) a. SN1 b. SN2 C. E1 ة d. E2 * "OH conc. H2SO4 Heatarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY