
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
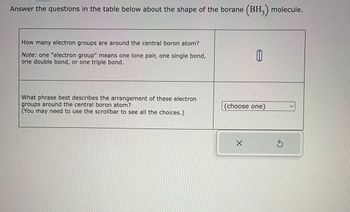
Transcribed Image Text:Answer the questions in the table below about the shape of the borane (BH3) molecule.
How many electron groups are around the central boron atom?
Note: one "electron group" means one lone pair, one single bond,
one double bond, or one triple bond.
What phrase best describes the arrangement of these electron
groups around the central boron atom?
(You may need to use the scrollbar to see all the choices.)
(choose one)
X
G
<
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B с D chemical symbol, chemical formula or Lewis structure H-C=C-H Fe He H-CEN: boiling point (Choose one) (Choose one) (Choose one) (Choose one Xarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. chemical symbol, substance chemical formula boiling point or Lewis structure H Н — С — Н - | A :N - О — Н (Choose one) | Н — С — Н H В Cr (Choose one) H H H | Н — С — С | C — О — С — н (Choose one) H H H D Ar (Choose one) :0 : :0 :arrow_forwardNonearrow_forward
- Pls help ASAP. Pls help on both PLSarrow_forwardAnswer the questions in the table below about the shape of the sulfur trioxide (SO3) molecule. How many electron groups are around the central sulfur atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central sulfur atom? (You may need to use the scrollbar to see all the choices.) 0 (choose one) X Śarrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. compound hydrogen-bonding force Between Between molecules of formula or Lewis molecules of the the compound and molecules of water? name structure compound? :0: || Н — С — о— Н yes yes formic acid no no yes О yes iodomethane CH,I no no H H yes yes dimethyl ether Н — С — - C -H no no H H O O ОО :o:arrow_forward
- Do not give handwriting solution.arrow_forwardThe table below includes the bond enthalpy (the energy required to separate the diatomic molecule into its atoms) and the bond length for each diatomic molecule. Diatomic Molecule Bond Enthalpy (kJ/mol) Bond Length (pm) Cl2 243 199 O2 498 121 N2 945 110 Identify the observed trend between bond enthalpy and number of shared electrons: as the number of electrons shared between two atoms increases, the bond enthalpy ( increases / decreases / remains unchanged ). Identify the observed trend between bond length and number of shared electrons: as the number of electrons shared between two atoms increases, the bond length ( increases / decreases / remains unchanged ).arrow_forwardPlease help me with this question (Explain). What would be the correct asnwers?arrow_forward
- Answer the questions in the table below about the shape of the nitrosyl choride (NOC1) molecule. How many electron groups are around the central nitrogen atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central nitrogen atom? (You may need to use the scrollbar to see all the choices.) 0 (choose one) Xarrow_forwardWhat is the total number of single bonds, double bonds, and triple bonds in the best Lewis structure o C2H2? O 3 single, 0 double, 0 triple O 2 single, 0 double, 1 triple O 2 single, 1 double, 0 triple O O single, 2 double, 1 triple O1 single, 2 double, 0 triplearrow_forwardplease draw a lewis structure, determine the # of electron groups around the central atom, and determine the geometry of the molecule. (please state both the name of the geometry and draw the molecular shape) molecule lewis structure electron pair geometry around the central atom(s) molecular shape around the central atom(s) CO2 H2S H2O2 NF3 SO2Cl2 NO2 C2H2 C2H4 C2H6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY