
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
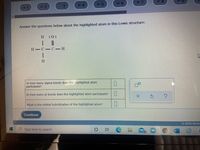
Transcribed Image Text:Answer the questions below about the highlighted atom in this Lewis structure:
H
:0:
Н— С —С-Н
In how many sigma bonds does the highlighted atom
participate?
In how many pi bonds does the highlighted atom participate?
What is the orbital hybridization of the highlighted atom?
Continue
O 2020 McGra
Type here to search
W
18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For NO3-: 1. Draw the Lewis Diagram 2. electron group geometry AND molecular geometry drawings & names 3. bond angle(s)? polar? resonance?arrow_forwardprovide the fllowing information including the lewis structure and the 3d sketch for NO2- and BrF3arrow_forwardVSEPR HOMEWORK Molecule Lewis Structure #e groups #lone-pair e Hybridization type VSEPR Formula (i.e Molecular Type) Geometric arrangement Molecular Shape Bond Angle Is the molecule Polar? Justify 3d Drawing PCI₂F₁arrow_forward
- Molecule or Polyatomic HOCI NO₂ N₂H4 H₂O₂ Hint: the oxygens are connected (CH3)2NH Lewis structure H¬O—CI: | : 0=N–6: H-N-N-H H H H-0-0-H H H H-C-N -C-H .. HHH Hybrid- ization AXE 3 sp AX₂E2 2 sp² AX₂E 3 sp³ AX3E 3 sp AX₂E2 3 AX3E Electron pair geometry Molecular shape tetrahedral bent trigonal planar bent tetrahedral trigonal pyramidal tetrahedral bent tetrahedral trigonal pyramidal Bond angles ~109.5° ~109.5° all ~109.5° all ~109.5° all ~109.5° Bond Polarity Molecular Polarity if the molecule is polar, add a dipole arrow if not polar, state so and why H H H²O-OH Н H H" H H H Н Cl H 'H Harrow_forwardPlease help with number 10. ICI4-arrow_forwardPredict the geometry around each highlighted atom.arrow_forward
- 5. Draw the # and molecular orbitals for the discrete and localized T bonds in the following structures. Indicate any polar- ization these bonds may have in your diagram through the shapes of the discrete bonding and antibonding orbitals. e 6. Show any plausible resonance structures for the molecules given in Exercises 1 and 5. If there are no plausible resonance structures, indicate this (note that the molecule corresponding to letter A is the same in each problem). 7. Discuss the hybridization in the C-C and C-H bonds of cyclopropane, given that the H-C-H bond angle is 118°. withara --- totrachlorida b dingl A. B. C. D. 3arrow_forwards it possible to draw a resonance structure with the lone pair? What is the hybridization of the heteroatom? Draw a valence bond orbital diagram to show the location of the lone pair (you do not need to draw any orbitals to show s bonds). In what orbital are the electron pairs located?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY