Question
Answer the following questions attached
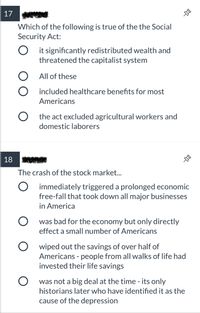
Transcribed Image Text:17
Which of the following is true of the the Social
Security Act:
it significantly redistributed wealth and
threatened the capitalist system
O All of these
O included healthcare benefits for most
Americans
O the act excluded agricultural workers and
domestic laborers
18
The crash of the stock market.
O immediately triggered a prolonged economic
free-fall that took down all major businesses
in America
was bad for the economy but only directly
effect a small number of Americans
O wiped out the savings of over half of
Americans - people from all walks of life had
invested their life savings
was not a big deal at the time - its only
historians later who have identified it as the
cause of the depression
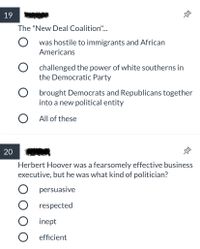
Transcribed Image Text:19
The "New Deal Coalition".
O was hostile to immigrants and African
Americans
O challenged the power of white southerns in
the Democratic Party
O brought Democrats and Republicans together
into a new political entity
O All of these
20
Herbert Hoover was a fearsomely effective business
executive, but he was what kind of politician?
persuasive
O respected
inept
O efficient
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps
