
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
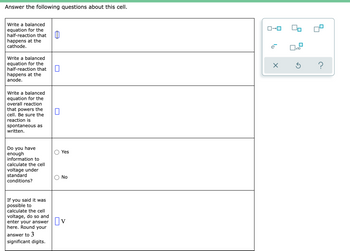
Transcribed Image Text:Answer the following questions about this cell.
Write a balanced
equation for the
half-reaction that
happens at the
cathode.
e
Write a balanced
equation for the
half-reaction that
happens at the
anode.
Write a balanced
equation for the
overall reaction
that powers the
cell. Be sure the
reaction is
spontaneous as
written.
Do you have
enough
information to
Yes
calculate the cell
voltage under
standard
No
conditions?
If you said it was
possible to
calculate the cell
voltage, do so and
enter your answer||V
here. Round your
answer to 3
significant digits.
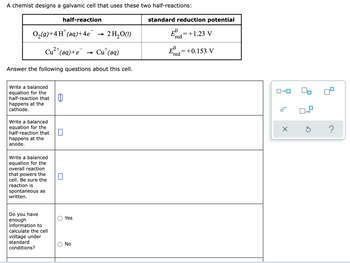
Transcribed Image Text:A chemist designs a galvanic cell that uses these two half-reactions:
half-reaction
standard reduction potential
0,(g)+4H"(aq)+4e
2 H,O(1)
=+1.23 V
(red
E=+0.153 V
red
2+
+
Cu"(aq)+e
- Cu' (aq)
Answer the following questions about this cell.
Write a balanced
equation for the
half-reaction that
happens at the
cathode.
Write a balanced
equation for the
half-reaction that
happens at the
anode.
Write a balanced
equation for the
overall reaction
that powers the
cell. Be sure the
reaction is
spontaneous as
written.
Do you have
enough
information to
calculate the cell
Yes
voltage under
standard
No
conditions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential Br,(1)+2e 2 Br (aq) :+1.065 V (red Cro, (aq)+4 H,O(1)+3e Cr(OH)3(s)+5 OH (aq) -0.13 V (red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. x10 Write a balanced equation for the half-reaction that happens at the anode.arrow_forwardHow is the amount of current flowing through an electrolytic cell related to the amount of product produced in the redox reaction? Match the items in the left column to the appropriate blanks in the sentences on the right. multiplied coulombs Avogadro's 1 mol Faraday's 1g ampers 6.022 x 10¹ mol volts divided The number of moles of electrons that have flowed in a given electrolysis cell can be determined by measuring the total charge that has flowed through the cell. If the amount of current flowing through the cell is by the time that the current flowed, the total charge that passed through the cell in that time can be found. The relationship between charge and the number of moles of electrons constant, which corresponds to the charge in is given by of of electrons. These relationships are used to solve problems involving the stoichiometry of electrolytic cells. Reset Helparrow_forwardAll of the following statements regarding electrochemical cells are true except All electrochemical cells require an applied electric current. In a voltaic cell the electrons flow from the anode to the cathode. In a voltaic cell, the half reactions are separated. The salt bridge maintains electricalcontact and charge neutrality.arrow_forward
- Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. 中 Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? O О If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 2 significant digits. Ον No Yes ローロ e ☐ x10 Garrow_forward0 A certain half-reaction has a standard reduction potential E 'red must provide at least 0.70 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the cathode of this cell. Note: write the half reaction as it would actually occur at…arrow_forwardO ELECTROCHEMISTRY Designing a galvanic cell from two half-reactions A chemist designs a galvanic cell that uses these two half-reactions: standard reduction potential half-reaction 2+ Fe (aq) Ed=+0.771 V %3D 3+ red Fe"(aq)+e Cro (aq)+4 H,O(1)+3e Cr(OH),(s)+5OH (aq) -0.13 V Ered nswer the following questions about this cell. Jrite a balanced quation for the alf-reaction that appens at the athode. rite a balanced uation for the If-reaction that ppens at the ode. ite a balanced uation for the erall reactionarrow_forward
- A chemist designs a galvanic cell that uses these two half-reactions: half-reaction MnO4(aq) + 2 H2O(l)+3e MnO2(s)+4OH(aq) Br₂(1)+2e →>> 2 Br (aq) standard reduction potential E = +0.59 V 'red E = +1.065 V 'red Answer the following questions about this cell.arrow_forwardA chemist designs a galvanic cell that uses these two half-reactions: MnO4(aq)+8H (aq) +Se Fe³+ (aq) +e Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. half-reaction Write a balanced. equation for the Answer the following questions about this cell. 0 - Mn² (aq) + 4H₂O(1) Fe (aq) standard reduction potential +1.51 V Ered=+0.771 V 20 "red =arrow_forwardZinc II Sulfate & Aluminum Chloride Half – Reaction in anode: Half- Reaction in cathode: Balanced cell reactionarrow_forward
- Write the half reaction for the anode and the cathode in the galvanic cell For Silver and Zincarrow_forwardA chemist designs a galvanic cell that uses these two half-reactions: standard reduction potential Exed half-reaction 2 H2O(1)+2e → H2(g)+2OH (aq) Ε red -0.83 V 2+ Zn(aq)+2e →>> Zn(s) E = = -0.763 V 'red Answer the following questions about this cell.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY