
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
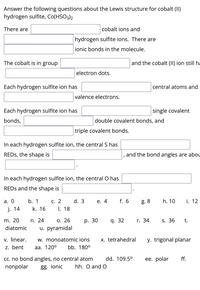
Transcribed Image Text:Answer the following questions about the Lewis structure for cobalt (II)
hydrogen sulfite, Co(HSO3)2
There are
cobalt ions and
hydrogen sulfite ions. There are
ionic bonds in the molecule.
The cobalt is in group
and the cobalt (II) ion still ha
electron dots.
Each hydrogen sulfite ion has
central atoms and
valence electrons.
Each hydrogen sulfite ion has
single covalent
bonds,
double covalent bonds, and
triple covalent bonds.
In each hydrogen sulfite ion, the central S has
REDS, the shape is
and the bond angles are abou
In each hydrogen sulfite ion, the central O has
REDS and the shape is
а. О
b. 1
С. 2
d. 3
е. 4
f. 6
g. 8
h. 10
i. 12
j. 14
k. 16
I. 18
m. 20
n. 24
О. 26
р. 30
q. 32
r. 34
S. 36
t.
diatomic
u. pyramidal
v. linear.
z. bent
w. monoatomic ions
x. tetrahedral
y. trigonal planar
aa.
0°
bb. 180°
ее. polar
cc. no bond angles, no central atom
nonpolar
dd. 109.5°
ff.
gg. ionic
hh. O and O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which one of the following molecules or ions has one lone pa ir on the central atom? (If there are 3 elements in the molecule, the first atom is the central atom and the others are bonded to it). AsCl 6 - BrO 4 - AsF 3 IO 2 - ClF 3arrow_forwardAnswer the following questions about the Lewis structure for calcium hydrogen carbonate, Ca(HCO3)2 There are calcium ions and hydrogen carbonate ions. There are ionic bonds in the molecule. The calcium ion has electron dots. Each hydrogen carbonate ion has central atoms and valence electrons. Each hydrogen carbonate ion has single covalent bonds, double covalent bonds, and triple covalent bonds. In each hydrogen carbonate ion, the central C has REDS, the shape is y and the bond angles are about In each hydrogen carbonate ion, the central O has REDS and the shape is а. О b. 1 С. 2 d. 3 е. 4 f. 5 g. 8 h. 10 i. 12 j. 14 k. 16 I. 18 m. 20 n. 24 О. 26 р. 30 q. 32 r. 34 S. 36 t. diatomic u. pyramidal v. linear z. bent y. trigonal planar w. monoatomic ions x. tetrahedral aа. 1200 bb. 180° Cc. no bond angles, no central atom nonpolar dd. 109.5° ее. polar ff. gg. ionic hh. O and Oarrow_forwardWhich of the following statements does not apply to a covalent bond? In the picture.arrow_forward
- 1. For each pair of elements listed determine if they will form an ionic (I) or covalent (C) bond. 2.General Properties of Ionic and Covalent Compounds (Use THIS LINK to complete the table) https://www.thoughtco.com/ionic-and-covalent-compounds-properties-3975966arrow_forwardPlease don't provide handwriting solutionarrow_forwardThe element selenium (Se) bonds with chlorine (CI) to make the formula SeCl_2. Chlorine is more electronegative than selenium. What is the name of this Covalent Compound?arrow_forward
- onic bonds are formed through the attraction of opposite charges to each other. With that said, O=O would have covalent bonds as there is no difference in charge between the two Oxygen atoms. What would be another example of an ionic bond?arrow_forwardUsing the answers to the questions attached answer the question: Why are compounds composed of integer ratios of elements?arrow_forwardGroup 1 elements have an average electronegativity of 0.84 (not including hydrogen). Group 17 elements have an average electronegativity of 2.99. These two groups often form bonds. Given this information, which kind of bond will they form? * Periodic Table of the Elements He Li Be C Ne Na Mg Al SI CI Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd in Sn Sb Xe Hf Ta Re Os Ir Pt Au Hg TL Pb Po At Rn Cs Ba Fr Ra Db Sg Bh Hs Mt Ds Rg Cn Nh FL Mc Lv Ts Og Rf Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Cf Es Fm Md No Lr Ac Th Pa Np Pu Am Cm Bkarrow_forward
- Which of these pairs of elements would be most likely to form an ionic compound? O Cl and I Al and Mg O Cl and Mg O Al and K O C and Sarrow_forwardAnswer the following questions about the Lewis structure for ammonium carbonate, (NHa)½CO3 There are ammonium ions and carbonate ions. There are ionic bonds in the molecule. Each ammonium ion has central atoms and valence electrons. In each ammonium ion, the central N has REDS, tH shape is (x), and the bond angles are The single carbonate ion has REDS and valence electrons. The carbonate ion has single covalent bonds, double covalent bonds, and triple covalent bonds. For the carbonate ion, the shape at the central C is and the bond angles are about а. О b. 1 С. 2 d. 3 е. 4 f. 5 g. 8 h. 10 i. 12 j. 14 k. 16 I. 18 m. 20 n. 24 О. 26 р. 30 q. 32 r. 34 S. 36 t. diatomic u. pyramidal v. linear z. bent w. monoatomic ions x. tetrahedral y. trigonal planar aа. 1200 bb. 180° ff. c. no bond angles, no central atom nonpolar dd. 109.5° ее. polar gg. ionic hh. O and Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY