
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
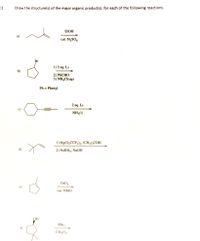
Transcribed Image Text:3.
Draw the structure(s) of the major organic product(s), for each of the following reactions.
ELOH
cat. H,SO,
Br
1) 2 eq. Li
b)
2) PHCHO
3) NH,C(ag)
Ph = Phenyl
2 oq. Li
c)
NH,(1)
1) Hg(O,CCF3)z, (CH,);COH
d)
2) NABH,, NAOH
cat. NMO
CH;C),
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is [OH-] of a solution consisting of 1.05 M NH3 and 0.98 M NH4NO3? Ka of NH4 + is 5.8 x 10-10 Group of answer choices 1.85e-5 1.85e5 5.37e-4 5.37e4arrow_forward3. Show the Steps necessay to transform the acid an te left into the compound on the Might. Be sure to use Soch. ->arrow_forwardIdentify the most acidic acid. a. ClCH2COOH b. FCH2CHOOH c. CH3COOH d. BrCH2COOH e. ICH2COOH f. can't be determinedarrow_forward
- Out of these choices, which ones are conjugate acid-base pairs?arrow_forwardDissociate your acid and base: (plsO +()s0 H* + Cl- + Ca2+ + 20H- – ni mots + H>OnolloB01 eirt gneesk Ga)edmun noitebixo pninpia We know that for each OH we need 1H* to neutralize it. So, in this case we will need 2H*. Balance the equation for H* and OH-. Remember each H* and OH- pair makes 1H2O. Also don't forget to balance the counter ions. 2H* + 2CI- + Ca2+ + 20H- → .norw+ 2H2O eos up to elquoarrow_forwardSodium hydroxide (NADH) is a cammonly used base with many apolicatons. A one-pmonth poducttion n the US is "about 2.25xl091bs. The density of NAOH is 2.130 a/cm. What is the volume ix km3 That is pnduced? Comnarrow_forward
- c. N2 Hs (aq): (ag): acid Obase conjugate acid conjugate base d. So. (aq) acıd base conjugate acidarrow_forwardDetermine the H*1. JOH], and pOH of a solution with a pH of 12.26 at 25 C. [H = %3D M (OH-| = pOH = Determine the H), JOH-|, and pH of a solution with a pOH of 6.69 at 25 "C. [H*) = pHarrow_forwardWhat are all the ionic and molecular species present in a 0.001 - M aqueous solution of C6H4OH(CO2H) in order of descending concentration?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY