
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Arrange the balanced biochemical equations for all the reactions in the payoff phase of glycolysis and the conversion of pyruvate to lactate.
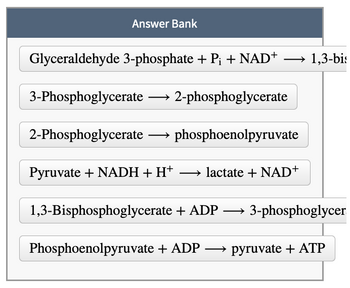
Transcribed Image Text:Answer Bank
Glyceraldehyde 3-phosphate + Pi + NAD+
-
3-Phosphoglycerate 2-phosphoglycerate
→ 1,3-bis
2-Phosphoglycerate →→→ phosphoenolpyruvate
Pyruvate + NADH + H+ →→→→lactate + NAD+
1,3-Bisphosphoglycerate + ADP →→→ 3-phosphoglycer
Phosphoenolpyruvate + ADP →→ pyruvate + ATP
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The mechanism of chymotrypsin can be viewed as a two-step process, acylation of the enzyme active site followed by a deacylation reaction. What accounts for the observed "burst" in rapid kinetic studies of the hydrolysis of N-acetyl-L-phenylalanine p-nitrophenyl ester by chymotrypsin? The rate of hydrolysis of the acyl-enzyme intermediate is faster than the rate of forming the acyl-enzyme intermediate. The rates of the acylation and deacylation reactions are equal. The rate of the acylation reaction is slower than the rate of the deacylation reaction. The rate of the acylation reaction is faster than the rate of the deacylation reaction.arrow_forwardFor the following enzymes (3-6) predict how the conditions will most likely affect the enzymes activity with one of the following and provide a 1 sentence explanation: a. increase activity b. decrease activity c. not likely to alter activity 3) alpha-ketoglutarate dehydrogenase complex binding to AMP when [AMP] is high. 4) phosphohexose isomerase binding NADH when [NADH] is high 5) phosphofructose-1 binding NAD+ when [NAD+] is high 6) pyruvate dehydrogenase complex binding to ATP when [ATP] is higharrow_forwardIn different organisms sucrose can be cleaved either by hydrolysis or by phosphorolysis. Calculate the ATP yield per mole of sucrose metabolized by anaerobic glycolysis starting with (a) hydrolytic cleavage and (b) phosphorolytic cleavage.arrow_forward
- Please provide an explanation for how to work through this practice problem: Using table 12.1, calculate the free energy change for the synthesis of ATP from cAMP and inorganic phosphate. (Note: cAMP is hydolyzed to AMP, and the free engery of hydrolysis for ATP and ADP is approximately equal.) Table 12.1: (Compound: Change in Free Energy in kJ/mol) cAMP: -50.4 Creatine phosphate: -43.3 ATP: -30.5 Glucose 6-phosphate: -13.9 AMP: -9.2arrow_forwardMalate dehydrogenase has a ΔGo′ΔGo′= 29.5 kJ/mol for the formation of oxaloacetate from malate yet plays a crucial role in the Citric Acid Cycle. Explain (briefly) how this enzyme with a very non-spontaneous ΔGo′ΔGo′can function well in a biochemical pathway.arrow_forwardWhat is meant by reciprocal regulation ? Name one compound that reciprocally regulates glycolysis and gluconeogenesis pathways . Which enzymes are regulated by this compound? What reactions are catalyzed by these enzymes (structures not required) ?arrow_forward
- What is the fate of the radioactive label when each of the following compounds is added to a cell extract containing the enzymes and cofactors of the citric acid cycle and the pyruvate dehydrogenase complex? P.S. A detailed steps explaination would be greatly appreciated. Also, any tips on how to solve such problems if possible? Is there any particular fate that C atoms of the R group for example, have, at the end of the cycle? I usually think of drawing out each step and solving but I believe that there must be a shorter, smarter way to do it and similar questions. Thank you!arrow_forwardProvide a reasonable step-wise mechanism for the reaction below, involving TPP as acoenzyme (can abbreviate the pyrophosphate as R), and invoking any general acids or base fromacetolactate synthase that you might need .arrow_forwardIn the skeletal muscle, in anaerobic conditions, glyceraldehyde 3-phosphate is converted into pyruvate during the payoff phase of glycolysis; and this pyruvate is reduced into lactate during lactic fermentation. Part 1-Write the 11 balanced biochemical equations corresponding to all the reaction steps leading to the conversion of glyceraldehyde-3-phosphate into lactate through glycolysis followed by lactic fermentation. Part 2-Write the net equation of the whole transformation process (i.e. with glyceraldehyde-3-phosphate as the starting substrate; and lactate as the end product).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON