
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer 7c
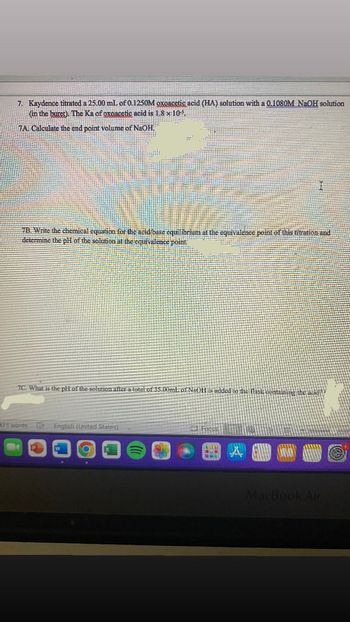
Transcribed Image Text:7. Kaydence titrated a 25.00 ml of 0.1250M oxoacetic acid (HA) solution with a 0.1080M NaOH solution
(in the buret). The Ka of oxoacetic acid is 1.8 x 105
7A Calculate the end point volume of NaOH,
I
7B. Write the chemical equation for the acid/base equilibrium at the equivalence point of this titration and
determine the pH of the solution at the equivalence point
7C. What is the pH of the solution after a total of 35.00ml of NaOH is added to the flask containing the acide
23 words English (United States)
Focus
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 36 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. The analytical ability of two lab technicians was compared by having each perform analyses for arsenic using the same method on samples having the same origin. For analyst A, s.d. = 0.12%, N=6; and for analyst B, s.d. = 0.06%, N-5. Do these results suggest a difference in the precision of the two chemists?arrow_forwardWhat is the unit of the answer?arrow_forwardLowest Highest 1A H2A Li Be Na Mg 3B 4B 5B K Ca Sc Ti V argon Rb Sr Y Zr Nb Mo Tc Ru Rh 3A 4A 5A 6A 7A He B CN OF NE 6B 7B 8B 1B 2B Al Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Pd Ag Cd In Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl **** Fr Ra Ac Rf Ha Using only the periodic table arrange the following elements in order of increasing ionization energy: neon, krypton, helium, argon . neon 8A Sn Sb Te I Xe Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Pb Bi Po At Rn Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur. Drag and drop your selection from the following list to complete the answer: krypton helium Previousarrow_forward
- Lowest 1A Highest 8A H2A Li Be Na Mg 38 48 58 68 7888 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha 3A 4A 5A 6A 7A He B C N O F Ne 18 2B Al Si P S Cl Ar Using only the periodic table arrange the following elements in order of increasing ionization energy: neon, helium, argon, radon. Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Cf Es Fm Md No Lrarrow_forwardAnswer choices: I II III IV Varrow_forwardk esc ~ 1. LiAlH4 2. Neutralizing work-up Select to Draw ! F1 F2 < O Q #つ 80 F3 NaCN, HCI Select to Draw $ 000 000 F4 % L F5 Problem 4 c MacBook Air < ( F6 8arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY