
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
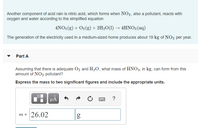
Transcribed Image Text:Another component of acid rain is nitric acid, which forms when NO2, also a pollutant, reacts with
oxygen and water according to the simplified equation
4NO2 (g) + O2(g) + 2H2O(1) → 4HNO3 (aq)
The generation of the electricity used in a medium-sized home produces about 19 kg of NO2 per year.
Part A
Assuming that there is adequate O, and H2O, what mass of HNO3, in kg, can form from this
amount of NO2 pollutant?
Express the mass to two significant figures and include the appropriate units.
HA
?
m = 26.02
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One step in the refining of titanium metal is the reaction of FeTiO3 with chlorine gas and carbon. Balance the equation for the reaction: FeTiO3(s) + Cl₂(g) + C(s) → TiCl4(1) + FeCl3(s) + CO(g).arrow_forwardCatalytic converters are the most common option for controlling emissions of polluting gases by internal combustion engines in automobiles, accelerating the conversion of nitrogen oxides into nitrogen and oxygen gases. A chemical reaction that can be used for the conversion of nitric oxide into non-polluting gases is the reaction of this gas with hydrogen, resulting in nitrogen and water gases, as shown in the chemical equation below: 2 NO(g) + 2 H2(g) → N2(g) + H2O(g) The kinetics of this reaction at a temperature of 1250 °C is represented in the following table, which indicates the influence of the concentration of the reactants on the reaction rate, obtained through three experiments conducted under the same conditions EXPERIMENTO [NO] (MOL/L) [H2] (MOL/L) V0 (MOL/Ls) 1 0,20 0,08 0,05 2 0,40 0,08 0,20 3 0,40 0,16 0,40 Determine the rate law for this chemical reaction, the rate constant, and its respective unit. Calculate the rate when the concentration of NO is 0.30…arrow_forwardUpon decomposing, a sample of sodium bicarbonate produces 0.0118 g of sodium carbonate, Na:CO. How many grams of water does it produce? (Use the balanced equation from question #1). 2NaHCO, → Na;CO, + CO2 + H;0arrow_forward
- An aqueous solution containing 5.01 g of lead(II) nitrate is added to an aqueous solution containing 5.63 g of potassium chloride. Enter the balanced chemical equation for this reaction. Be sure to include all physical states. balanced chemical equation: Pb(NO3)₂(aq) + 2KCl(aq) - → → PbCl₂ (s) + 2KNO, (aq) What is the limiting reactant? potassium chloride lead(II) nitrate The percent yield for the reaction is 89.8%. How many grams of the precipitate are formed? precipitate formed: 3.78 Taking into account the percent yield, how many grams of the excess reactant (the reactant that is not limiting) remain? excess reactant remaining: 2.53 Incorrect g 09 garrow_forwardA mixture of barium chloride and sulfuric acid yield the precipitate barium sulfate and hydrochloric acid according to the double replacement reaction: H,SO, (aq) + BaCl, (aq) → Baso4 (s) + 2 HCI (aq) A large quantity of barium chloride is used to ensure that the sulfuric acid is the limiting reactant. If 39.5 grams of barium sulfate are produced, what mass of H2SO4 must be dissolved in the initial aqueous solution? g [Number in standard notation; round your answer to one decimal place] The following molar masses may or may not be needed, but are provided to save you time: • barium sulfate = 233 g/mol • sulfuric acid = 98.0 g/molarrow_forwardIf sodium peroxide is added to water, elemental oxygen gas is generated, consider this unbalanced equation: Naz O2 (s) + H2 O(1) → NAOH(aq) + 02(9) Suppose 5.80 g of sodium peroxide is added to a large excess of water. What mass of oxygen gas will be produced? Mass =arrow_forward
- Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. Ca, (PO,), (s) + SiO, (s) + C(s) → CaSio, (s) + P, (s) + CO(g) 2 alo Ararrow_forwardThe balanced chemical equation is P₄(s) + 6 Cl₂(g) → 4 PCl₃(g). What is the mass in grams of phosphorus trichloride that can be formed from 226.0 grams of chlorine gas based on the balanced chemical equation?arrow_forwardA fictitious metal called Oscium (MM = 72.4 g· mol) reacts with nitrogen gas according to the following reaction: 6 Oc (s) + N2(g) + 2 OC3N(s)arrow_forward
- For the following reaction, 6.80 grams of water are mixed with excess chlorine gas. The reaction yields 15.1 grams of hydrochloric acid. chlorine (g) + water (1) →→→→hydrochloric acid (aq) + chloric acid (HCIO3) (aq) What is the theoretical yield of hydrochloric acid ? || What is the percent yield of hydrochloric acid ? grams %arrow_forwardIn a lab, a grade 11 chemistry student used 0.0123 mol of solid magnesium metal. The student proceeds to burn the magnesium metal in a Bunsen burned with an excess amount of oxygen gas to produce 0.41g of magnesium oxide based on the following balanced equation: 2Mg(s) + 02(g) —> 2MgO(s). The theoretical amount of magnesium oxide should be what?arrow_forwardA sample of 49.0 g of tetraphosphorous decoxide (P,O10) reacts with 49.0 g of water to produce phosphoric acid (H, PO,) according to the following balanced equation. P,010 + 6 H,O → 4H,PO, Determine the limiting reactant for the reaction. H,PO, O H,0 O P,O10 Calculate the mass of H, PO, produced in the reaction. mass of H, PO4: Calculate the percent yield of H, PO, if 37.5 g of H, PO, is isolated after carrying out the reaction. percent yield:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY