
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:HAnnouncements - 2021 Sprin x
S Microsoft Word - 3- Homewo X
1o Chem101
GBPJPY 153.074 v -0.04% U X
A app.101edu.co
M Inbox (581) - andainecampbe x
Question 2 of 21
Submit
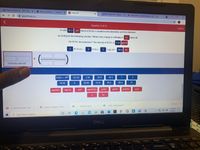
A solid 30.2 cm block of KCIO: is heated in the laboratory and decomposes
according to the following reaction. What is the change in enthalpy in kJ when all
the KCIO: decomposes? The density of KCIOS is 2.34 g/cm
2 KCIO:(s) – 2 KCI(s) +
3 0:(g) AH = -89.4
kJ
STARTING AMOUNT
6.022 x 10
122.55
2.34
-25.8
-42.4
1
3
74.55
32.00
-51.6
30.2
-89.4
-11
mol O2
cm
mol KCIO.
g KCI
g/cm
g KCIO,
g O2
mol KCI
J
kJ
Show all
Andaine's.docx
study questions.docx
3- Homework and.pdf
outline 2 chapter 5.docx
1:16 PM
4/5/2021
P Type here to search
DELL
Home
PgDn
Delete
Pgup
Apsert
PrtScr
F12
F11
F10
F9
Num
Lock
F8
F6
F5
Backspace
F4
F2
F3
&
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chem 101 -> app.101edu..co E Apps Dashboard Gmail.com YouTube MyCollege- MyV.. The A8.05 g sample of an unknown salt (MM = 116 82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the 22 temperature of the salt and water is 23 72 °C. After the salt has completely dissolved, the temperature of the solution is 28 54 "C 1 of 1 point earmed Given that 158.05 g of solution increased in temperature by 4.82 C. how much heat was gained by the solution? Assume the specific heat of the solution is the same as water 4.184 J/g-"C. O of 1 point earned 10 attempts remaining What is the total heat for the dissolution reaction of the 8.05 g of salt? O of 1 point earned 19amemenreaning How many moles of the unknown salt were used in the reaction? 0 of 1 point earned What is the enthaipy.change rn kUmo of sat for the dissolution reaction? O of 1 point earned 9 Type here to search 个arrow_forwardThe enthalpy changes for the reaction CO2(g)+3H2(g)= CH3OH(g)+ H2O(g) exceeds - 52kJ/mol True or False? Show steps Pleasearrow_forward14arrow_forward
- 8. From the following heats of combustion, A,H° =-726.4 kJ mol A,H° =-393.5 kJ mol CH;OH(/) + 3/202(g) → CO2(g) + 2H20(!) C(graphite) + O2(g)→ CO2(g) H2(g) + ½O2(g) → H20(!) A,H° =-285.5 kJ mol Calculate the enthalpy of formation of methanol (CH3OH) from its elements: C(graphite) + 2H2(g) +½O2(g) → CH;OH(!)arrow_forwardWhich on the three delta H’s was the largest? Explain why.arrow_forward- IrmityR Name: Enthalpy Practice #1 Consider the reaction for production of water vapor: 2H, +0, → 2H,0+572KJ / mol a. As the reaction proceeds, energy is absorbed kreleased. b. This reaction can be classified asexothermicendothermic G. The sign of the enthalpy term (AH) would be positive Knegative.)C Pacitve. @>absorbed B> exothermic #2 Consider a chemical system where the reactants have a total internal energy of 658KJ and the products have a total internal energy of 458 kJ. a. This reaction is exothermie/ endothermic. b. The value of the enthalpy of reaction is Vo ko kJ/mol. (circleor -) c. The reactants/producs are more thermodynamically stable. #3 Consider the photosynthesis reaction: 2870KJ/mol +6CO, +6H,0→C¸H„O, +60, a. This reaction isexothermi/ endothermic. b. The value the enthalpy of reaction is+/- kJ/mol. Thermochemistry-Stoichiometry SHOW WORK for credit # 4 How many moles of glucose would be produced if 6 moles of CO, and 6 moles of H,0 are reacted? mol CaH1206 #5 What…arrow_forward
- The standard enthalpy of formation (AH°;) of C2H4(g) is +52.4 kJ/mol. What is the coefficient of H2 in the formation reaction of C2H4(g)? Enter your answer as a whole number in the answer box.arrow_forwardpls help out thanks chemmarrow_forwardMolar Masses A = 290.41 D = 250.53– 9 %3D E = 75.44 9 %3D mole mole %3D mole G = 117.05 X = 10.93 Z = 50.01 mole mole mole If 18.23 g A, according to the balanced thermochemical equation below, how much heat (in kJ) is absorbed? 5 A+6 D→ 5 Z +5G AH = 515 kJ Please input your answer to the second decimal place Type your answer...arrow_forward
- Please calculate Ccal for both runs!arrow_forward14 Write the thermochemical equation for the combustion of liquid butane (C.H10). AH C,H10 0= -147.6 CO2 ( = -393.5 H,O (s) = kJ H.C -241.8 (3)arrow_forwardapp.101edu.co Dashboard M Gmail.com YouTube MyCollege- MyVC.. TH ! Apps 23 Consider the hypothetical thermochemical equation 3 A+B-2 C for which AH = 71.5 kJ/mol. If you have 5.30 moles of A. how much heat would be absorbed? 0 of 1 point earned 10 attempts remaining If the reaction absorbs 345 kJ of heat, how many moles of C would be produced? O of 1 point earned 10 attempts remaining What would H be for the reaction 2 C-3 A+ B? O of 1 point earned 10 attempts remaining What would H be for the reaction 9 A+38-607 0 of 1 point earned 10 attempa remainng Which one of the following reactions would have an enthalpy change of-2×AH? Here AH refers to the enthalpy change of the original reaction. e O of 1 point earned Type here to search C Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY