
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For each measured sample from Table 2, use the ICE table method to determine the molar equilibrium concentration of Fe3+, SCN-, and FeSCN2+. (For this one, if you can do just one example that would be great, you don't have to do all of them!)
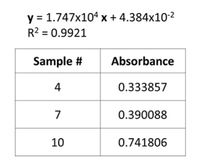
Transcribed Image Text:y = 1.747x10ª x + 4.384x10-2
R2 = 0.9921
Sample #
Absorbance
4
0.333857
7
0.390088
10
0.741806
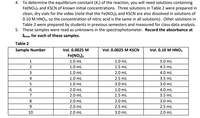
Transcribed Image Text:4. To determine the equilibrium constant (K.) of the reaction, you will need solutions containing
Fe(NO3)3 and KSCN of known initial concentrations. Three solutions in Table 2 were prepared in
clean, dry vials for the video (note that the Fe(NO:)3 and KSCN are also dissolved in solutions of
0.10 M HNO3, so the concentration of nitric acid is the same in all solutions). Other solutions in
Table 2 were prepared by students in previous semesters and measured for class-data analysis.
5. These samples were read as unknowns in the spectrophotometer. Record the absorbance at
Amax for each of these samples.
Table 2
Sample Number
Vol. 0.0025 M
Vol. 0.0025 M KSSCN
Vol. 0.10 M HNO,
Fe(NO,)3
1
1.0 ml
1.0 ml
5.0 mL
1.0 mL
1.5 ml
4.5 mL
1.0 ml
2.0 ml
4.0 mL
4
1.0 ml
2,5 mL
3.5 mL
1.0 ml
3.0 ml
3.0 ml
2.0 mL
1.0 ml
4.0 mL
7
2.0 ml
1.5 mL
3.5 mL
8.
2.0 ml
2.0 ml
3.0 ml
2.0 mL
2,5 ml
2.5 ml
10
2.0 ml
3.0 ml
2.0 ml
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the correct equilibrium constant expression for the balanced reaction shown below? PC13 (9) + PBr3 (9) PCl2Br (g) + PCIBr2(g) [PCl3] [PBr3] [PCl₂ Br][PCIBr₂] [PCIBr₂][PCl₂Br] [PCl3] [PBr3] [PCIBr₂][PCl3][PBr3] [PCl₂Br] [PCIBr₂]²[PCl₂Br]² [PCl3] [PBr3] [PCl3][PBr3] [PCIBr₂]²[PCl₂ Br]² O a. a O b.b OC.C O d.d O e.e a b C d earrow_forwardHow did the change of stress (adding or removing reactants or products) cause a shift in the equilibrium system of the solutions (in which direction) use an example. Use trial one as an examplearrow_forwardExpress the equilibrium constant for the following reaction.arrow_forward
- A student ran the following reaction in the laboratory at 431 K: PC15 (9) PC13 (g) + Cl₂ (g) When she introduced 2.98 moles of PC15 (g) into a 1.00 liter container, she found the equilibrium concentration of PC15 (9) to be 2.94 M. Calculate the equilibrium constant, Kc, she obtained for this reaction. Kc = Submit Answer [Review Topics] [References] Use the References to access important values if needed for this question. Show Hint Retry Entire Group 9 more group attempts remaining 2 Previous Next or n er etarrow_forwardInitial (M) Change (M) Equilibrium (M) Consider the reaction of SO₂ and O₂ described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. 2 SO₂(g) + O₂(g) = 2 SO₂(g) NEXT A 2.00 L reaction vessel was filled 0.0432 mol SO₂ and 0.0296 mol O₂ at 900 K and allowed to react. At equilibrium, the concentration of SO, was found to be 0.0175 M. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. -0.00875 0.0209 0 0.00875 2SO₂(g) Question 20 of 33 2.00 0.0216 0.0432 0.0148 0.0296 0.0041 O₂(g) 0.0175 2 0.0129 -0.0175 0.0061 RESET -0.0350 0.0257 2SO₂(g)arrow_forwardHydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition: compound concentration at equilibrium 0.86 M Cl, 0.30 M HC1 1.9 M Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = 0arrow_forward
- Consider the equilibrium 3Al + 2Baq 2Cg. Which of these is the correct expression for Q? Q = PC2 + [1]3 + [B]2 Q= (PC2) / ([1]3 + [B]2) Q = (2PC) / (3[1] + 2[B]) Q = (PC2) / ([1]3 x [B]2) Q = (2PC) / (3[1] x 2[B])arrow_forwardHydrogen and chlorine react to form hydrogen chloride, like this: H₂(g) + Cl₂(g) - 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition: compound concentration at equilibrium H₂ 0.26M Cl₂ HCI Kc Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. = 1.4M 0 0.97 M 0 x10 Xarrow_forward056575718snapshotld=13245488&id-5636490008takeld=4bfba59b09f0e5b& Q Search this course X References Use the References to access important values if needed for this question. Consider the following reaction where K, = 4.55x10 at 723 K: H N2(g) +3H2(g)2NH3(g) 1008 If the three gases are mixed in a rigid container at 723 K so that the partial pressure of each gas is initially one atm, what will happen? A-Z Indicate True (T) or False (F) for each of the following 1. A reaction will occur in which NH (g) is consumed. 2. Kn will increase. 3. A reaction will occur in which N, is consumed 4. Q is less than K. 5. The reaction is at equilibrium. No further reaction will occur 4. Q versus K: This is group attempt 1 of 5 Autosaved at 1:06 PM Back Next 1:06 PM PRA x 11 9/24/2019 Lhp delete 12 prt sc f11 insert KAarrow_forward
- For the following reaction : H2 (g) + I2 (g) 2HI (g) it was found that a 13.0L vessel at equilibrium contained 0.852 mol H2, 0.361 mol I2, and 2.99 mol of HI. A) Write an expression for Kc. B). Determine the value of Kc for this reaction.arrow_forwardGive correct answer please..don't use Ai for answering thisarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY