
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pls do fast and i will give like for sure
Try to give solution in typed form
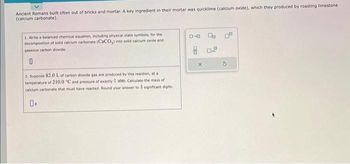
Transcribed Image Text:Ancient Romans built often out of bricks and mortar. A key ingredient in their mortar was quicklime (calcium oxide), which they produced by roasting limestone
(calcium carbonate).
1. Write a balanced chemical equation, including physical state symbols, for the
decomposition of solid calcium carbonate (CaCO3) into solid calcium oxide and
gaseous carbon dioxide.
0
2. Suppose 82.0 L of carbon dioxide gas are produced by this reaction, at a
temperature of 210.0 "C and pressure of exactly 1 atm. Calculate the mass of
calcium carbonate that must have reacted. Round your answer to 3 significant digits.
0-0
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- T LI (256) YouTube X b Answered: A chemical engineer is X www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnus2sAfexSL1600dL1a21X_MSqqptw-p1SkOK39SVTrZeBN4xlCuFmxOf2Tp3q?1oB... Type here to search X (256) ali hamza baloch - YouTube X ● ENTROPY AND FREE ENERGY Calculating dG from dH and dS 2NO(g) + Cl₂ (g) → 2NOC1(g) A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 111.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. C(s) + 2C₁₂(g) → CC₁₁ (g) Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether,…arrow_forwardPlease show work A tums tablet pulverized (powdered) and 0.4265 g of this power was mixed with 9.00 mL 1.000 M HCl. This mixture was back titrated with 0.1000 M NaOH with phenolphthalein as indicator. It required 8.25 mL of 1000 M NaOH to reach the end point. Compute % CaCO3 in the tums sample and % error.arrow_forwardtech Please don't provide handwritten solutionarrow_forward
- ! A C Escape Chemistry 106! Type here to search Final Exam Equation Sheet.pdf: X https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG40h2E11 NuVCU4MS9 120% The Ksp values for four salts are shown in the picture. The solubility of THREE of * the salts can be directly compared without doing any math. Arrange the three salts in order of increasing solubility. Example: If the three salts that can be compared are the first three and they are already in order of increasing solubility, enter ABC. Solid A: Solid B: Solid C: Solid D: Your answer + Try again! A₂Br3 X₂F5 Y2Cl3 Z₂F3 3.4 x 10-² 7.8 x 10-5 1.9 x 10-16 6.4 x 10-8 Ⓒ↓|||| Barium sulfite (BaSO3) is a sparingly soluble salt. You need to dissolve as much * || a W 65°F Mostly cloudy J ABP 8:28 PM 5/14/2023arrow_forwards Systems Qu. Study P.T Systems F. [Review Topics] [References)] Use the References to access important values if needed for this question. When the following molecular equation is balanced using the smallest possible integer coefficiets, the values of these coefficients are: magnesium nitride (s) + water (I) magnesium hydroxide (aq) + ammonia (aq) Submit Answer Retry Entire Group 9 more group attempts remaining Ne Show Hint Email Instructor Saarrow_forwardFast pls solve this question correctly in 5 min i will give u like for surearrow_forward
- O KINETICS AND EQUILIBRIUM Calculating the solubility of an ionic compound when a... Calculate the solubility at 25 °c of BaCro in pure water and in a 0.0170M BaCl₂ solution. You'll find ê data in the ALEKS Data tab. sp Round both of your answers to 2 significant digits. solubility in pure water: 17/0 solubility in 0.0170 M BaCl₂ solution: 0²/ 0 x10 X □ × ロ 0/3 8arrow_forwardChrome File Edit View History ← → C ||| O CHEMICAL REACTIONS Solving limiting reactant problems in solution A ALEKS-Carly Bruskewitz - Le X www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslka... Q M Explanation Check New Tab X 3 Suppose 2.22 g of barium acetate is dissolved in 200. mL of a 57.0 m M aqueous solution of sodium chromate. Calculate the final molarity of acetate anion in the solution. You can assume the volume of the solution doesn't change when the barium acetate is dissolved in it. Be sure your answer has the correct number of significant digits. O X O + 67 0/5 ★ Carly V ?圖 olo Ar e be ta D her ble earch © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility hat fo ● . hat is hat isarrow_forwardhelp a girl outtt?arrow_forward
- Help 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwarda Gateway Support -...arrow_forwardWhat is the solubility of nitrogen gas at 25*Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY