
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
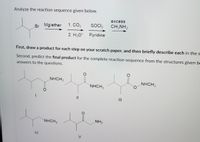
Transcribed Image Text:Analyze the reaction sequence given below.
excess
Br Mg/ether
1. CO2
SOCI,
CH;NH2
2. H3O' Pyridine
First, draw a product for each step on your scratch paper, and then briefly describe each in thes
Second, predict the final product for the complete reaction sequence from the structures given be
answers to the questions.
NHCH3
NHCH3
NHCH,
II
NHCH3
NH2
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the missing reactant in the following reaction? Select one: DA N OB. III O C. OD. II ? N&OC₂H₁ HOC₂H5 CHoli || & si "G OCH OCHSarrow_forwardNO ₂ Provide the product / reagants product 2 1. Fe, HCI 2. NUNO₂, HLI 3. Cu CN Na ₂ Cr 07 H ₂ O, H ₂ SO 4 1. NaN 3 2. H₂, Pt / reagants to complete the reaction sequences 2 2 OH ? ? 1. Ao 2. Socl₂, pyr. 2 2arrow_forwardPlease don't provide handwriting solutionarrow_forward
- 6. Complete the following reactions by filling what is left out; either the reactants, starting material, or the major product. a. b. C. CI 1. Excess NaNH, 2. H₂O 3.HgSO4 4. NaBH₁, NaOH 1.NAOMe 2.03 3. Zn, H* Coquearrow_forwardRank from most to least reactive in an EAS reaction H 1. II. O=0 Br ||| A.IV>II>I> ||| B.II> IV> | > | C.II>IV>I> ||| D.I>>> IV IVarrow_forward12. For each reaction below, the starting mass/volume of each reagent is provided as well as the final mass of product isolated. Calculate the theoretical and percent yield for each reaction. a. b. OH OH + l MW 122 1.50 g CI MW 141 d 1.21 g/mL 0.250 mL MW 60.1 7.40 g 1. PhMgBr MW 181 905 mg 2. HCI H3PO4 OH Ph MW 164 1.86 g Ph MW 260 547 mg + H₂Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY