
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
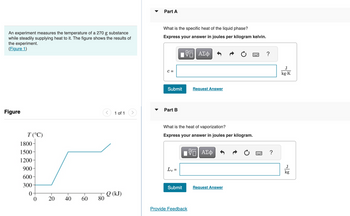
Transcribed Image Text:An experiment measures the temperature of a 270 g substance
while steadily supplying heat to it. The figure shows the results of
the experiment.
(Figure 1)
Figure
T (°C)
1800-
1500-
1200
900-
600-
300-
0
0
20
40
60
80
1 of 1 >
Q (kJ)
Part A
What is the specific heat of the liquid phase?
Express your answer in joules per kilogram kelvin.
C =
Submit
Part B
ΠΫΠΙ ΑΣΦ
L₁ =
What is the heat of vaporization?
Express your answer in joules per kilogram.
Submit
Request Answer
Provide Feedback
ΫΠΙ ΑΣΦ
Request Answer
?
?
J
kg.K
J
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- In an electrically heated home, the temperature of the ground in contact with a concrete basement wall is 14.0 °C. The temperature at the inside surface of the wall is 21.4 °C. The wall is 0.12 m thick and has an area of 7.7 m2. Assume that one kilowatt hour of electrical energy costs $0.10. How many hours are required for one dollar's worth of energy to be conducted through the wall? Number Units the tolerance is +/-2%arrow_forwardAn aluminum cup with mass 0.3 kg holds 0.35 kg of water. Both the cup and the water have a temperature of 20.°C. If a 0.75-kg piece of copper at 85.°C is added to the cup, what is the final equilibrium temperature in °C? You may assume that the cup, water, and copper are well insulated from anything else. (What can I do to solve this equation?)arrow_forwardIf you have 0.276 m of water at 25.0 °C in an insulated container and add 0.112 m³ of water at 95.0 °C, what is the final temperature T; of the mixture? Use 1000 kg/m² as the density of water at any temperature. T; = °Carrow_forward
- A lead rod has a length of 60.6 m when the temperature is 12.4 degrees celsius. What is its length when the temperature is 51.2 degrees celsius? Note: Your answer must be in meters, however, do not include the unit, just enter the magnitude that correspond to the final length in meters. Round your answer to 2 decimal points Coefficient of linear expansion for lead = = 29 x 10-6 °C-¹arrow_forwardA glass window pane, 196 cm high, 58 cm wide and 0.4 cm thick, has thermal resistance of 6.41x10-3 m2 °C w-1. Calculate the rate of heat transfer through the window if the temperature of outer and inner surfaces is 26.3 °C and 21.3 °C, respectively. Select one: 3293 W 911 W 887 W O 5325 Warrow_forwardA gas with an initial temperature of 940 °C undergoes the process shown in the figure. (Figure 1) Figure p (atm) 3 2 1- 0 0 100 200 2 300 V (cm³) Part A What type of process is this? Isochoric. Isobaric. Isothermic. Submit ✓ Correct Part B What is the final temperature in °C? Express your answer using two significant figures. T= 3400 °C Submit V= Previous Answers Part C ✓ Correct Previous Answers How many moles of gas are there? Express your answer to two significant figures and include the appropriate units. Submit HÅ A 29 10-4 mol Previous Answers Request Answer P Pearson ?arrow_forward
- On a linear X temperature scale, water freezes at -101.0°X and boils at 415.0°X. On a linear Y temperature scale, water freezes at -78.00°Y and boils at -30.00°Y. A temperature of 57.00°Y corresponds to what temperature on the X scale? Number i Unitsarrow_forwardA superhero is building a suit of armor made of Vibranium (Vb). To mold the headpiece of this suit, he need to heat 1 kg of the metal into its forging temperature. Its forging temperature must be at least 70% of its melting point in Kelvin (MPyB = 3297 K). Assuming that the melting point and the forging temperature of Vb can be achieved, answer the following questions. a. Determine the specific heat capacity (J/kg-°C) of Vb if it loses 519.2775 kJ as it is being cooled from its melting point down to its forging temperature. b. The newly forged headpiece is then submerged in 2.5 L of liquid water initially at room temperature. Since the headpiece is still at its forging temperature right before it was submerged in water, some of the water vaporized in the process. What is the volume (L) of the remaining liquid water if the headpiece and the remaining water are in thermal equilibrium at 50 °C?arrow_forwardIn an electrically heated home, the temperature of the ground in contact with a concrete basement wall is 11.9 °C. The temperature at the inside surface of the wall is 19.0 °C. The wall is 0.13 m thick and has an area of 7.5 m². Assume that one kilowatt hour of electrical energy costs $0.10. How many hours are required for one dollar's worth of energy to be conducted through the wall? Number i Unitsarrow_forward
- An aluminum cup with mass 0.26 kg holds 0.30 kg of water. Both the cup and the water have a temperature of 15.°C. If a 0.55-kg piece of copper at 80.°C is added to the cup, what is the final equilibrium temperature in °C? You may assume that the cup, water, and copper are well insulated from anything else. (What can I do to solve this? What equations can I use?)arrow_forwardA 2-meter-long steel rod has a diameter equal to 2.54 cm. By how much does the rod’s length change in meters if it’s heated from 318 degrees Kelvin to 460 degrees Kelvin? _________ m . You should get a really small answer, feel free to use exponential notation to express it.arrow_forward1000 800 600 400 200 10 20 30 40 50 60 70 80 90 -200 400 Time in seconds Look at the graph above. How much did the temperature change from t = 30 to t = 60 seconds? %3D AT = unit Select an answer v This substance has a specific heat of 0.13 cal/g°C. If you have 34 grams of this substance, how much heat was added from t = 30 to t = 60 seconds? Q = unit Select an answer v Temperature In Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON