
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
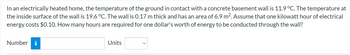
Transcribed Image Text:In an electrically heated home, the temperature of the ground in contact with a concrete basement wall is 11.9 °C. The temperature at
the inside surface of the wall is 19.6 °C. The wall is 0.17 m thick and has an area of 6.9 m². Assume that one kilowatt hour of electrical
energy costs $0.10. How many hours are required for one dollar's worth of energy to be conducted through the wall?
Number i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 39% maximum efficient coal-fired power plant needs to produce 600 MW of power. A. To achieve maximum efficiency, if the waste water is dumped into a 13 degree Celsius lake, what temperature must the steam be heated to? B. Since coal contains 27 kJ of energy per gram, how many kg of coal must be burned in a day?arrow_forwardThe amount of heat per second conducted from the blood capillaries beneath the skin to the surface is 210 J/s. The energy is transferred a distance of 1.7 x 10-3 m through a body whose surface area is 1.6 m². Assuming that the thermal conductivity is that of body fat, determine the temperature difference between the capillaries and the surface of the skin. Number i Unitsarrow_forwardA small metal cube with a thermal mass mc and an initial temperature 0 is dropped into a container of water that is actively maintained at a constant temperature 0w. The cube quickly comes to rest on the bottom surface of the container. The bottom surface is maintained at a constant temperature 0 (note that this is different from 0w). The thermal resistance between the cube and the water is Rcw while the thermal resistance between the bottom surface of the container and the cube is RCB. The temperature of the cube is denoted by 0c. a) Draw the system schematic indicating the assumed directions of the heat transfer rates. Label all the nodes and system parameters. b) Derive the governing equation for the temperature of the cube, 0c. c) Where does to appear in the system schematic and how does it affect the governing equations? d) Calculate the steady-state temperature of the cube, css, assuming the following system parameters: 0o = 21°C, 0B = 6°C, 0w = 0°C, Rcw = 2°C/W, and RCB = 4°C/Warrow_forward
- D-E please!arrow_forwardQuestion 10. A detective has been called to the scene of a crime where a dead body has just been discovered. The detective arrives at 9:00 AM and begins to investigate. Immediately, the temperature of the body is taken and is found to be 90.3°F. The detective checks the programmable thermostat and finds that the room has been kept at a constant 68.0°F for the past 3 days. After the evidence from the crime scene is collected, the temperature of the body is taken once more and found to be 89.0°F. This last temperature reading was taken exactly one hour after the first one. The following day, the detective is asked "At what time did our victim die?" Assuming that the victim's body temperature was normal (98.6°F) prior to death, what should the detective answer?arrow_forwardThe heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) Figure p (kPa) 600 400 200- 0 0 0.025 0.050 V (m³) Part A Determine T₁, T₂, and T₁. Enter your answers numerically separated by commas. Express your answer using two significant figures. T₁ T₂. T₂ = 600,1800,1200 K Submit Previous Answers ✓ Correct Part B Determine AEth. W. and Q for 1-2. Enter your answers numerically separated by commas. Express your answer using two significant figures. AEth. Ws. Q = 3.0x104.1.3x104.4.3x104 J Submit ✓ Correct ▾ Part C Previous Answers Determine AEth. W. and Q for 2-3. Enter your answers numerically separated by commas. Express your answer using two significant figures. AEth. Ws. Q= Submit ▾ Part D ΠΑΣΦ Request Answer I ? Jarrow_forward
- A typical coal-fired power plant burns 300 metric tons of coal every hour to generate 2.7 × 106 MJ of electric energy. 1 metric ton = 1000 kg; 1 metric ton of coal has a volume of 1.5 m3. The heat of combustion of coal is 28 MJ/kg. Assume that all heat is transferred from the fuel to the boiler and that all the work done in spinning the turbine is transformed into electric energy.a. Suppose the coal is piled up in a 10 m × 10 m room. How tall must the pile be to operate the plant for one day?b. What is the power plant’s efficiency?arrow_forwardAssume that Q₁ = -15.0 J and Q2 = -80.0 J in (Figure 1). For help with math skills, you may want to review: Substituting Numbers into Mathematical Expressions For general problem-solving tips and strategies for this topic, you may want to view a Video Tutor Solution of Heat engine details. Figure p (kPa) 400 200 0 0 [] 2₁ 100 200 < 22 1 of 1 -V (cm³) Part A What is Wout for the heat engine shown in the figure? Express your answer with the appropriate units. Wout= Submit Request Answer Part B Value QH = What is QH for the heat engine shown in the figure? Express your answer with the appropriate units. Units Value Units ? ?arrow_forwardQuestion 5 Specific Heat of Solid Substances Substance D Aluminum B silver; because it is a transition metal with the lowest specific heat value C Iron Copper Tin Given the specific heat information presented, which substance would make the BEST material to create a pan for cooking and why? D Specific Heat (J/kg°C) 899 443 385 213 Tin - because it has a low specific heat, which means it conducts heat the best. Aluminum - because it has a high specific heat, which means it conducts heat the best. Copper - because it has a mid-level specific heat, which means it is a good insulator. Iron - because it has a mid-level specific heat, which means it is a good conductor.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON