
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
What is the answer
Transcribed Image Text:GROUPS
RESOURCES
COURSES
Atomic Structure and Periodic Table Quiz
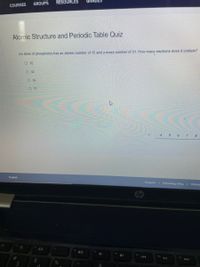
An atom of phosphorus has an atomic number of 15 and a mass number of 31. How many neutrons does it contain?
O 15
O 42
O 16
O 31
4 5 6 7
English
Support Schoology Blog I PRIVA
ho
#3
トト
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardNow draw two waves on your own sheet of paper. Predict what would happen if they arrive at the same spot at the same time (also known as "in-phase"). What would be the effect? Enter your answer here Save Answerarrow_forwardA ALEKS-Alasia Fuqua-Learn X VHL Central | Dashboard x G when you take the logarithm doy x + ← C www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBjuJnuCDtT6kRBabGFF3MOAKZ_UVX0N202gOiJQTdIGDtYib1Jke3qJJtY-n0BOXd4GOr80JmhB6zbOrS5lo8W-LPce6pq?10Bw7QY = Apple Music O CHEMICAL REACTIONS Writing the net equation for a sequence of reactions 0 X G mg to g-Google Search There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC₂(s)+2H₂O(g)-C₂H₂(9)+ Ca(OH)₂(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6C₂H₂(9)+3 CO₂(g) + 4H₂O(g)-5CH₂CHCO₂H(9) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced. Explanation X Check X Do DO 1/5 S Feb 1 Alasi BEEN olo Ar 7:09 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY