
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Use the table below the solve the ff thermal expansion problems:
- An aluminum flagpole is 33 m high. By how much does its length increase as the temperature increases by 15 °C?
- At 20°C, a brass cube has edge length 30 cm. What is the increase in the surface area when it is heated from 20°C to 75°C?
3. What is the volume of a lead ball at 30.0°C if the ball’s volume at 60.0°C is 50.00 cm3?
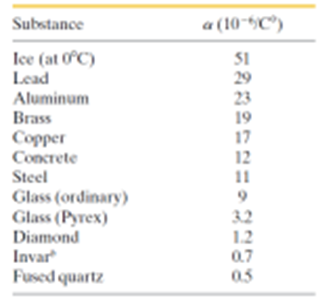
Transcribed Image Text:Substance
Ice (at 0°C)
Lead
Aluminum
Brass
Copper
Concrete
Steel
Glass (ordinary)
Glass (Pyrex)
Diamond
Invar
Fused quartz
a (10-5C)
51
29
23
19
17
12
11
9
1.2
0.7
0.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Needs Complete solution with 100 % accuracy.arrow_forwarda.Plot P vs T graph for the constant V using Excel. Choose the independent variable T along the x-axis and P along the y-axis. Is the graph linear? If so, use a linear fit and write down thebequation of the best-fit straight line. If not, use a power law fit of y = xn. What is the value of n? Based on the fit, are the variables directly or inversely proportional? Temperature T (K) Pressure 300 35.1 350 40.7 400 46.5 450 52.4 500 58.3 550 64.1 600 69.9 650 75.8 b.Plot P vs. V graph for constant T using Excel. Choose the independent variable w (for V, which is ~ w) along the x-axis and P along the y-axis. Is the graph linear? If so, use a linear fit and write down the equation of the straight-line fit. If not, use a power law fit of y = xn. What is the value of n? What does it say about the relation between pressure and volume of a gas at constant temperature? Are the variables directly or inversely proportional? Width Pressure 10 75.8 10.5 72.1 11.5 65.6…arrow_forwardThe cylinder in the figure(Figure 1) has a moveable piston attached to a spring. The cylinder's cross-section area is 10 cm?, it contains 0.0040 mol of gas, and the spring constant is 1500 N/m. At 20 °C the spring is neither compressed nor stretched. Figure 1 of 1> 1500 N/m www A- 10 cm How far is the spring compressed if the gas temperature is raised to 140 °C?arrow_forward
- Please only answer parts vi and vii. The answers are shown in the square brackets next to the questions.arrow_forwardWill continue to run if you leave the test. Remaining Time: 29 minutes, 29 seconds. ¥ Question Completion Status: QUESTION 1 The initial volume of a sample of gas is 400.0 mL . At constant pressure, the temperature of the gas is increased from 22.0 °C to 30.0 °C?. What will be the final volume of the gas ? TT T Arial 3 (12pt) Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save 888 F5 F4 F3 F2 % 5 6 4arrow_forwardA lead rod has a length of 60.6 m when the temperature is 12.4 degrees celsius. What is its length when the temperature is 51.2 degrees celsius? Note: Your answer must be in meters, however, do not include the unit, just enter the magnitude that correspond to the final length in meters. Round your answer to 2 decimal points Coefficient of linear expansion for lead = = 29 x 10-6 °C-¹arrow_forward
- Can you please answer number 3 and show all of the stepsarrow_forward3 of 4 A stainless-steel-bottomed kettle, its bottom 25 cm in diameter and 1.6 mm thick, sits on a burner. The kettle holds boiling water, and energy flows into the water from the kettle bottom at 800 W. Review I Constants I Periodic Tabl Part A What is the temperature of the bottom surface of the kettle? Thermal conductivity of stainless steel is 14 W/(m K). Express your answer using four significant figures. VO AEO ? T = 102.65 °C Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Review your calculations and make sure you round to 4 significant figures in the last step. Ne Provide Feedbackarrow_forwardThe specific heat of liquid water is 4190 J/kg. K. ▼ Part A What is the molar specific heat of liquid water in J/mol. K? Give your answer as a multiple of R, rounded to the nearest half integer. Express your answer as a multiple of R to the nearest half integer. Cmol = 9 R Submit Correct Part B Previous Answers Equal moles of liquid water and helium gas are heated at constant pressure from the same initial temperature to the same final temperature. By what factor is the entropy increase of the water larger than the entropy increase of the helium? Express your answer to two significant figures. AS water ASHe Submit 17| ΑΣΦ Request Answer ?arrow_forward
- 4.0 g of oxygen gas, starting at 18 °C, follow the process 1 → 2 shown in the figure(Figure 1). Figure Pi 0 0 1 V₁ 2 1 of 1 Part A What is temperature T2 (in °C)? Express your answer with the appropriate units. T₂ = Submit μA Value Provide Feedback Request Answer Units ?arrow_forwardSolve it correctly please. I ll rate accordinglyarrow_forwardA graph of temperature versus time for a substance that has a mass of 2.00 kg is shown below. The sample is at a temperature of-10.0 degrees celsius and initially in the solid phase. Heat is added to the sample at a constant rate of 20.0 kj/minute. B& Care at a temperature of 119 degree Celsius. Answer the following questions: 1. What is the melting point of this substance? 2. What islare the phase/phases that exists/exist during the process from A to B and during the process from B to C? 3. Use the graph to calculate the specific heat for this substance in solid phase. 4.Use the graph to calculate the latent heat of fusion for this substance. Temperdture cc) B 120 + 100 80 60 40+ 20 + 12 4 6. 10 %3D 13 -20 Time (min) %3D F00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON