
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
An air-conditioning system is to be filled from a rigid container that initially contains m1 kg of liquid R-134a at T°C. The valve connecting this container to the airconditioning system is now opened until the mass in the container is m2 kg, at which time the valve is closed. During this time, only liquid R-134a flows from the container. Presuming that the process is isothermal while the valve is open, determine the final quality of the R-134a in the container and the
total heat transfer.
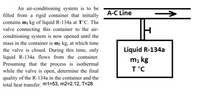
Transcribed Image Text:An air-conditioning system is to be
filled from a rigid container that initially
A-C Line
contains m1 kg of liquid R-134a at T°C. The
valve connecting this container to the air-
conditioning system is now opened until the
mass in the container is m2 kg, at which time
the valve is closed. During this time, only
Liquid R-134a
liquid R-134a flows from the container.
Presuming that the process is isothermal
while the valve is open, determine the final
quality of the R-134a in the container and the
total heat transfer. m1=53, m2=2.12, T=28
mı kg
T°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A 0.08-m rigid tank initially contains saturated water vapor at 180 °C. The tank is connected by a valve to a supply line that carries steam at 1400 kPa and 400 °C. Now the valve is opened, and steam is allowed to enter the tank. Heat transfer takes place with the surroundings such that the temperature in the tank remains constant at 180 °C at all times. The valve is closed when it is observed that one-third of the volume of the tank is occupied by liquid water. Find (a) the amount of steam that has entered the tank, and (b) the amount of heat transfer. 1400 kPa Steam 400°C Saturated vapor Qout V=0.08 m T, = 180°Carrow_forwardA piston cylinder contains 1.25 kg water at 25°C with a constant load on the piston such that the pressure is 300 kPa. A nozzle in a line to the cylinder is opened to enable flow to the outside atmosphere at 100 kPa and 25°C. The process continues until 85% of the initial mass has flowed out. At this point, the temperature of water increased by 10°C. Assume that the process is done in an Water isobaric manner. Write the mass and energy balance equations and calculate the work (kJ) and heat (kJ) involved in the process if the exit velocity is 40 m/s. For compressed liquid, assume the substance is a saturated liquid at the given temperature.arrow_forwardAn insulated piston-cylinder device contains 4.5 L of saturated liquid water at a constant pressure of 200 kPa. Water is stirred by a paddle wheel while a current of 20 A flows for 42 min through a resistor placed in the water. If 36% of the liquid is evaporated during this constant pressure process and the paddle-wheel work amounts to 280 kJ, determine the voltage of the source. Also, show the process on a P-v diagram with respect to saturation lines. H₂O = constant W P= Wh 4 Activate Winiarrow_forward
- A 0.3m³ rigid tank initially contains saturated liquid water at 200°C. A valve at the bottom of the tank is opened until half of the mass in saturated liquid form is withdrawn from the tank. The temperature is maintained constant. Determine the amount of heat transfer during this process. Sat. liquid T = 200°C V = 0.3 m³arrow_forwardA 0.78 m³ tank initially contains water at 260°C and a quality of 0.81. A valve at the top of the tank is opened and allows for saturated vapor at 260°C to leave the tank. The tank is maintained isothermally during this process until the tank is filled with only saturated vapor at 260⁰ C. What is the mass of steam that leaves the tank during this process? How much heated is added to the tank during this process. KJ kgarrow_forwardA 0.68 m3 rigid tank contains refrigerant-134a initially at 100 kPa and 40 percent quality. Heat is now transferred to the refrigerant until the pressure reaches 400 kPa. Determine: Final temperature the mass of the refrigerant in the tank the amount of heat transferred.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY