
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I am confused about where the 54.1 and 21.1 numbers came from because the scale is marked 2ppm to 10 ppm. I want to be able to di this myslef on a test . Can someone give me step by step instructions?
Transcribed Image Text:106rthw mqq S to bleitqu
sasd
snovbe art to omod nois
CHU
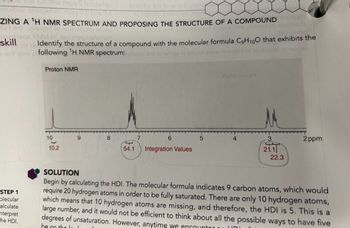
ZING A ¹H NMR SPECTRUM AND PROPOSING THE STRUCTURE OF A COMPOUND
sqed to noitubong shutu
ями
STEP 1
colecular
alculate
interpret
the HDI.
zu enamuostuner
skill
Identify the structure of a compound with the molecular formula C₂H₁00 that exhibits the
ad otni er following 'H NMR spectrum:alloon or lo amor ni bnuol onow nisqari
Proton NMR
10
haped
10.2
8
6
T
54.1 Integration Values
5
AMM notor
3
21.1
ar
22.3
2ppm
SOLUTION
Begin by calculating the HDI. The molecular formula indicates 9 carbon atoms, which would
require 20 hydrogen atoms in order to be fully saturated. There are only 10 hydrogen atoms,
which means that 10 hydrogen atoms are missing, and therefore, the HDI is 5. This is a
large number, and it would not be efficient to think about all the possible ways to have five
degrees of unsaturation. However, anytime we encounter
UD
be on the l
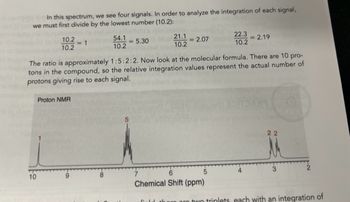
Transcribed Image Text:In this spectrum, we see four signals. In order to analyze the integration of each signal,
we must first divide by the lowest number (10.2):
10.2
10.2
10
<-1
Proton NMR
54.1
10.2
-5.30
21.1
10.2
2.07
The ratio is approximately 1:5:2:2. Now look at the molecular formula. There are 10 pro-
tons in the compound, so the relative integration values represent the actual number of
protons giving rise to each signal.
22.3
10.2
6
Chemical Shift (ppm)
-2.19
22
3
there are two triplets, each with an integration of
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An infant ibuprofen suspension contains 100 mg/ 5.0mL suspension. The recommended dose is 10 mg/kg body weight. How many milliliters of this suspension should be given to an infant weighing 21 lbs?arrow_forwardY Part C aspirin with [H3O+] = 6.5 x 10 M Express your answer using two significant figures. Y VE ΑΣΦ Submit Part D Request Answer IVE ΑΣΦ 1 Submit 9 pancreatic juice with [H3O+] = 3.5 x 10 M Express your answer using two significant figures. → O Provide Feedback Request Answer ar Ű ? ? Copyright © 2023 Pearson Educatioarrow_forwardTubes 1-4 are standards with known mass and known volume of 200µL. Tubes 5-7 are unknowns that have been diluted 1/100 and volume used 200μL. How do you work out the concentration of tubes in g/L using a standard curve to work out the initial mass of unknowns. Tube No. 1 2 3 4 5 6 7 Protein amount (ug) 0 50 100 200 Absorbanc e (750nm) 0 0.32 0.5005 0.824 0.612 0.805 0.69arrow_forward
- A 32.75 g sample of a solid is placed in a flask. Toluene, in which the solid is insoluble, is added to the flask so that the total volume of solid and liquid together is 53.00 mL. The solid and toluene together weigh 58.54 g. The density of toluene at the temperature of the experiment is 0.864 g/mL. Part A What is the density of the solid? Express your answer in grams per milliliter to three significant figures. ? p = 25.93 g/mLarrow_forwardA solution was created by dissolving 7.399 g of calcium chloride in 54.2 mL of water (d = 1.00 g/mL). What is the boiling point of this new solution? The ko for water is 0.52°C/m. The boiling point of pure water is 100.0°C. Round your answer to 2 decimal places.arrow_forwardHow to convert 0.50 dL to mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY