
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:References
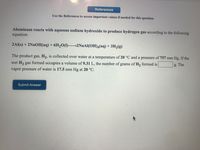
Use the References to access important values if needed for this question.
Aluminum reacts with aqueous sodium hydroxide to produce hydrogen gas according to the following
equation:
2Al(s) + 2NAOH(aq)+ 6H2O(1)–→2NAAI(OH)4(aq) + 3H2(g)
The product gas, H2, is collected over water at a temperature of 20 °C and a pressure of 757 mm Hg. If the
wet H2 gas formed occupies a volume of 9.31 L, the number of grams of H, formed is
g. The
vapor pressure of water is 17.5 mm Hg at 20 °C.
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of hydrogen gas (in mL) at 707 mm Hg and 42 °C can be produced when 2.1 g of CaCO3(s) are put into an Erlenmeyer flask containing 225 mL of 0.261 M HCl(aq) ?1 CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)arrow_forward1.) This reaction occurs in large test tube at 32.0oC: 2 Al (s) + 3 H2SO4(aq) ---> 6 H2 (g) + Al2(SO4)3 (aq) A tube going through the stopper of the test tube feeds into a graduated cylinder that has been inverted in a tub of water. At the end of the reaction, the total pressure of gas in the inverted cylinder is 435. torr, and the volume of gas collected is 455. mL. What mass of aluminum must have reacted? P.s- please show how you got it. I get confused with itarrow_forwardA) A 3.50 liter metal cylinder contains only ethane gas, C2H6. If the pressure in the container is 2.50 atm and the temperature is 25.0 C what mass of ethane is in the cylinder? B) The ethane in the metal cylinder is mixed with 30.0 grams of oxygen and the mixture is ignited. Write a balanced formula equation for the combustion of ethane. Determine the limiting reagent Calcilate the mass of each reactant and product present at the completion of the reaction C) The heat of combustion of ethane is1560kj/mol. If all of the heat produced by the previously described reaction is transfered to 10.000kg of water with an initial temperatre of 18.0 degrees celcius what is the final temperature of water? The specifice heat of water = 4.184J/(gxC)arrow_forward
- If boron hydride, B4H10, is treated with pure oxygen, it burns to give B2O3 and H2O: 2 B4H10(s) + 11 O2(g) ®4 B2O3(s) + 10 H2O(g) If a 0.050-g sample of the boron hydride burns completely in O2, what will be the pressure of the gaseous water in a 4.25-L flask at 30.0°C?arrow_forward5 & 6 go together.arrow_forward04/22/ 2021 ot art e b NAME Kouri KulRu DATE SECTION POSTLABORATORY ASSIGNMENT 1. Calculate the theoretical percentage of water for the following hydrates. (a) manganese(II) monohydrate, MnSO4 • H2O (b) manganese(II) tetrahydrate, MNSO4 • 4H2O 2. An unknown hydrate, AC• XH»O, has a mass of 1 .00 g before heating, and 0.738 g atter heating. What is the experimental percentage of water in the hydrate? ombvl lo slmo noihesilla logataW If the anhydrous compound (AC) has a molar mass of 101 g/mol, what is the water of crystallization (X) and the formula for the hydrate (AC XH,O)?ouphut suf (snoilgo) ai sh Formula of hydrate AC H20 Water of crystallizationarrow_forward
- When zinc metal is added to hydrochloric acid, the following reaction takes place Zn(s) + 2 HCl(aq) → ZnCl₂(aq) + H₂(g) Calculate the volume of H₂ gas collected over water at 30.0 °C when 71.9 g of zinc is added to excess hydrochloric acid if the total pressure is 695 torr. The vapor pressure of water at 30.0 °C is 31.8 torr.arrow_forwardThe human body burns glucose C6H12O6 for energy according to this chemical reaction:→+C6H12O66O2+6CO26H2O. The products of the reaction are carbon dioxide CO2 and water H2O. Interestingly, all of the carbon dioxide and much of the water exits the body through the lungs: on every breath, the average person exhales 500.mLof air, which is typically enriched to 4% CO2 and 5% water vapor by volume. In short, when a person loses weight by dieting, the weight that is lost actually departs his body as a gas, every time he exhales. Each kilogram of body fat lost requires exhaling about 3.0kg of carbon dioxide. Calculate how many breaths it takes an average person to "exhale" 2.00kg of fat. Round your answer to the nearest thousand. You'll need to know that the density of CO2 is 2.0/kgm3arrow_forwardSuppose that a 26.4mL volume of HCl solution reactions completely with 55.0 mL of aqueous Na2CO3. The volume of CO2 formed is 148 mL at 23 degrees Celsius and 732 mmHg. What is the molarity of the HCl solution?arrow_forward
- What is the molarity of a HClHCl solution if the reaction of 215. mLmL of the HClHCl solution with excess CaCO3CaCO3 produces 12.2 LL of CO2CO2 gas at 725 mmHgmmHg and 18∘C∘C?arrow_forward1) a) Sulfuric acid, the industrial chemical produced in the greatest quantity each year, is prepared by the reaction of sulfur in the presence of oxygen and water as described in the balanced chemical equation below. Calculate the volume, in ml, of 18.0 M sulfuric acid solution that can be prepared from the reaction of 1850 g of sulfur with 6.50x10'mL oxygen gas at 25°C and 0.965atm and plenty of water. 25(solid) + 302 (gas) + 2H20(liquid) _-→ 2H2SO4(aqueous) b. Which substance is the limiting reactant? Explain how you know.arrow_forwardWhat volume of carbon dioxide gas (in mL) at 835 mm Hg and 37 °C can be produced when 2.4 g of CaCO3(s) are put into an Erlenmeyer flask containing 108 mL of 0.152 M HCl(aq) ?1 CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY