
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:**Aluminum Chloride (AlCl₃) in Organic Reactions**
Aluminum chloride, AlCl₃, is used in a variety of organic reactions as a Lewis acid. Although it adopts a more complex geometry in the solid-state, consider it as a single molecule (i.e., in the gas phase).
**Valence Electrons and Lewis Structure**
AlCl₃ has 24 total valence electrons. Draw the Lewis structure for AlCl₃ that minimizes the formal charges on all atoms.
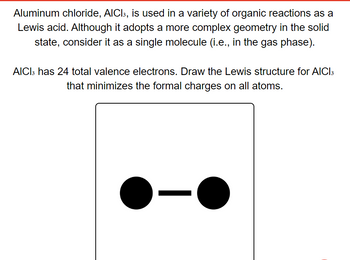
**Visual Aid**
Below is a partial visual representation:
- The image depicts two black circles connected by a single black line.
- This likely represents a simplified model of the AlCl₃ molecule.
(Note: The image provided is an incomplete representation and might not accurately depict the final Lewis structure of AlCl₃.)
Use this information and your understanding of Lewis structures to draw the complete Lewis structure of AlCl₃ with minimized formal charges.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the SO 3 molecule. How many valence electrons does the molecule have? How many lone pairs of electrons are on the central atom? How many lone pairs of electrons in total are on the substituent atoms? How many resonance structures can be drawn for the molecule? If the ion doesn't exhibit resonance, indicate "1" as only one structure can be drawn for the molecule.arrow_forward5. There are three possible ways to linearly connect the atoms for an anion whose chemical formula is [CNO]. They are [CNO], [NOC], and [NCO]. Draw a Lewis structure (showing all bonds, lone pairs, and formal charges) for each of the three possible ions. Which is the most stable arrangement? (Hint: the most stable structure will be the one with the most octets, fewest and lowest formal charges, etc., as we discussed in class.)arrow_forwarda. Write a Lewis structure that obeys the octet rule for the following species. Assign the formal charge for the central atom of XeO4. If multiple resonance structures exist, use one that does not involve an expanded valence. Formal charge: b. Write a Lewis structure that obeys the octet rule for the following species. Assign the formal charge for the central atom of clO3¯. If multiple resonance structures exist, use one that does not involve an expanded valence. Formal charge:arrow_forward
- Draw one valid Lewis structure (including all lone pair electrons and any formal charges) for the anion (CH2CN)−. HCCN Harrow_forwardConsider the following ion: BrO3−. a) Show the full electron configuration for Br. b) Draw the most correct Lewis structure for BrO3− and briefly explain why your Lewis structure is correct. c) If the structure is stabilised by resonance, draw at least one of the possible resonance forms. If it is not stabilised by resonance, briefly explain why. d) What is the electronic geometry of BrO3−? What is its molecular shape? e) Does BrO3− have a dipole moment? Briefly justify your answer. f) On average, would you expect IO3− to have longer or shorter bonds than BrO3−? Briefly explain your answer. g) Which of the following molecules would you expect to have the lowest vapour pressure? Briefly explain your choice. (IMAGE WITH POSSIBILITIES) h) What is the molecular formula for Compound C? What is the empirical formula for Compound C?arrow_forwardThe value of Ka1 of hypophosphorous acid, H3PO2, is nearly the same as the Ka1 of phosphoric acid, H3PO4. Draw a Lewis structure for phosphoric acid that is consistent with this behavior. Draw the Lewis structure with the formal charges minimized. Do not include formal charges in your structure. Include hydrogen atoms and nonbonding electrons in the structure.arrow_forward
- For a molecule of XeCl3H a) Draw the Lewis structure then predict the shape of the compound and draw the shape of the compound b) Determine if the molecule is polar or nonpolar and explain how you made that determination. If there is a dipole moment, draw it.arrow_forwardAn incompicie Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of -1. N-C-C-H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges. H N=c=c-Harrow_forwardThe formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for the sulfite ion, SO3²-. The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen atoms starting from the left for each structure. Formal Charge S 01 Use the References to access important values if needed for this question. 02 03 S KNENEN :0: B A I 2- 2. The best Lewis structure for SO3²- is A :0: C Previous Nextarrow_forward
- Draw regular Lewis structures (no need to use dashed lines and wedges) for each of the following molecules and indicate which exception, if any, to the octet rule is found in each of them: SCI6, CH3, XeF4, BBR3.arrow_forwardThe compound below (Cl2O2) plays a role in ozone depletion in the stratosphere, where it serves as a reservoir of Cl atoms that catalyze the destruction of O3. Draw a Lewis structure for Cl2O2 based on the arrangement of atoms shown above.arrow_forwardWhen drawing the Lewis structure of the HCC13 molecule, the structure should represent a total of 26 valence electrons. What is the best description of the complete Lewis structure of the molecule? Select one: AH atom should be in the center with single bonds to each Cl atom and a double bond to the C atom. A Cl atom should be in the center with a single bond to the C atom and a single bond to the H atom. AC atom should be in the center with a double bond to the H atom and double bonds to each Cl atom. AC atom should be in the center with single bonds to each Cl atom and a single bond to the H atom.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY