
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
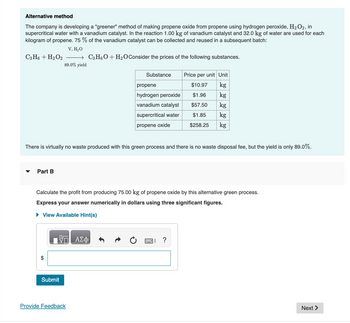
Transcribed Image Text:Alternative method
The company is developing a "greener" method of making propene oxide from propene using hydrogen peroxide, H₂O2, in
supercritical water with a vanadium catalyst. In the reaction 1.00 kg of vanadium catalyst and 32.0 kg of water are used for each
kilogram of propene. 75% of the vanadium catalyst can be collected and reused in a subsequent batch:
V, H₂O
→ C3H6O + H₂O Consider the prices of the following substances.
89.0% yield
C3H6 + H₂O2
▼
Part B
There is virtually no waste produced with this green process and there is no waste disposal fee, but the yield is only 89.0%.
ΠΙΑΣΦ
Substance
Submit
propene
hydrogen peroxide
vanadium catalyst
supercritical water
propene oxide
Calculate the profit from producing 75.00 kg of propene oxide by this alternative green process.
Express your answer numerically in dollars using three significant figures.
► View Available Hint(s)
Provide Feedback
Price per unit Unit
$10.97
kg
$1.96
kg
$57.50
kg
$1.85
kg
$258.25 kg
?
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction for the oxidation of NH3 is given as: 4 NH3 + 5 024 NO + 6H₂O Under certain conditions the reaction will proceed at 29.8% yield of NO. How many grams of NH3 must be used to react with excess oxygen to yield 70.5 g of NO? Hint: 70.5 g of NO is the actual yield. 21.0 134 237 2.37 None of these responses apply.arrow_forwardIf the yield of the reaction is 76.7%, what is the actual yield of chlorine?arrow_forwardAmmonia (NH3) is naturally produced through the decomposition of organic material as wellas being high in wastewater discharged to natural waters. In the environment it is oxidizedto form nitrite (NO2-), and nitrite is then oxidized to form nitrate (NO3-) in a process callednitrification. The balanced equations are given below:2NH3 + 3O2 → 2NO2- + 2H+ + 2H202NO2- + O2 → 2NO3-a) Assuming complete oxidation, what is the theoretical oxygen demand to convert100mg/L of ammonia to nitrate (i.e., how much oxygen is necessary)? Report youranswer as mg/L of O2.Answer: 376 mg/L O2b) This conversion process makes water more acidic. With reference to the equations forpH, explain why this occursarrow_forward
- The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH)3(s)-Al₂O3(s) + 3 H₂O(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 Al₂O3(s)+3 C(s)-4 Al(s) + 3 CO₂(g) Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. Explanation Check 80 E5 ローロ X On F6 S MacBook Air F7 DII FB FO A Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access F10 A F11 ola Ar 00arrow_forward1. Chlorine has two isotopes, 35Cl and 37Cl. Which isotope has a higher natural abundance? Show your calculations. U 2. Extraction of aluminum from Bauxite ore starts with the following reaction: a Al₂O3 + b NaOH → c NaAlO₂ + d H₂O (a) Balance the equation, i.e., specify the values of a. b. c, and d. (b) Assuming a theoretical reaction yield, at what mass will Al₂O3 no longer be a limiting reagent in a process that also uses 40 ko of sodium hydroxide?arrow_forwardIn a closed system, equal amounts of ammonia and oxygen react to produce nitrogen monoxide and water. What is the limiting reactant? NH3 4NH3 + 502 - 4NO + 6H,0 NO H20 OO Oarrow_forward
- Atomic Weight of a Metal from Hydrogen Gas Production Objective: To produce hydrogen gas by reaction of a metal with an acid. To use the ideal gas equation to determine the atomic weight of the metal. Background: Many metals react with hydrochloric acid to produce hydrogen gas and metal ions. In this Lab you will use Magnesium: Mg(s) + 2 H+(aq) → H2(g) + Mg2+(aq) The moles of hydrogen gas produced can be determined using the Ideal Gas equation, PV = nRT. From the moles of hydrogen and the stoichiometry (reacting ratios) of the reaction, it will be possible to calculate how many moles of metal reacted. From this, the atomic weight of the metal will be determined. When a gas is collected over water, it becomes saturated with water vapor and the total pressure of the mixture in the buret equals the partial pressure of the gas plus the partial pressure of the water vapor. The partial pressure of water vapor depend on temperature. Finally, the total pressure of gas…arrow_forwardWrite a balanced chemical equation for the formation of 1 mol of Cr₂O3(s) from Cr and O2 in their standard states. (Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. If no reaction occurs, leave all boxes blank and click on Submit.) ✓+3/20₂(g) ✓- →Cr₂O3(s) 2Cr(s) The equation of the formation of Cr₂O3 (s) from the elements: 2 Cr(s) + 3/2 O2(g) → Cr₂O3(s) b What is the enthalpy change if 3.1 g of chromium is oxidized to Cr₂O3(s)? AfH for Cr₂O3 (s) is -1134.7 kJ/mol Enthalpy change = Submit Submit Answer OCT 30 kJ Try Another Version tv ♫ 10 item attempts remaining 5 LIZA O Cengage Learning Cengage Technical Support Correct 144 B MacBook Pro * 23 Ⓡ E Previous Next Email Instructor Save and Exit Uarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY