
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
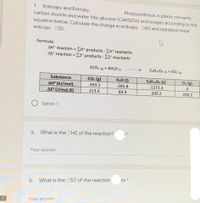
Transcribed Image Text:1. Enthalpy and Entropy.
carbon dioxide and water into glucose (C6H1206) and oxygen according to the
Photosynthesis in plants converts
equation below. Calculate the change in enthalpy DHO and standard molar
entropy OSD.
Formula:
AH° reaction = EH° products -EH° reactants
AS° reaction = Es° products - ES° reactants
%3D
6CO2 (R) + 6H20 m
C6H1206 (s) + 602 (e)
Substance
CO2 (g)
H20 (1)
COH1206 (s)
O2 (B)
AH° (kJ/mol)
AS (J/mol.K)
-393.5
-285.8
-1273.3
213.6
69.9
209.2
209.2
Option 1
а.
What is the OHO of the reaction?
Your answer
ts *
b.
What is the DSO of the reaction
Your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- The reaction quotient is Q=1.6×10-26 Part B What pH is needed to produce this value of Q if the concentration and pressure values are [Br2]=2.50×10−4M , [Br−]=11.65M, [SO42−]=9.50M, and PSO2=3.50×10−5atm ? Express your answer numerically to two decimal places.arrow_forwardAccelerated Stability Testing Data Determine the expiration date of a drug product with the initial drug concentration of 50 mg/tablet using the following accelerated stability testing data: log Temp Rate Constant (1/min) 1/T (oC) 70 1.73E-05 80 3.38E-05 90 6.45E-05 Reaction Kinetics - Accelerated Stability Testing log In k Rate Constant Temp (oC) Temp (к) (1/min) (1/K) k 70 1.73E-05 80 3.38E-05 90 6.45E-05arrow_forwardConsider the following reaction and its equilibrium constant: 12(g) 21(g) Kp = 0.209 atm A reaction mixture contains 0.89 atm 12 and 1.77 atm I. Which of the following statements is TRUE concerning this system?arrow_forward
- (please type answer fast).arrow_forwarddraw the alkane strucutre based off the IR spectrum, 1H NMR spectrum, and 13C NMR spectrum for this compound.arrow_forwardFill up the two missing values inside the two circles knowing that the total reaction volume is 2mL. 1) Dilution fraction 2) Water Don't include units in your answer. Membrane Brillant blueR 1g/L Dilution Sample suspension fraction (μL) (μL) 1) Control ? 0 50 2) 1/200 ? 10 50 3) 1/100 0.01 20 50 4) 1/50 ? 40 50 5) 1/20 100 50 Phosphate pH11, 0.2M (ml) 1 1 1 1 1 Water (mL) 0.95 ? ? ?arrow_forward
- When the following equation of a redox reaction in acidic solution is properly balanced, what are the coefficients for Cr2O72–, Fe2+ H+, Cr3+, Fe3+, and H2O, respectively? __Cr2O72– + __Fe2+ + __H+ --> __Cr3+ + __Fe3+ + __H2O (A) 1, 3, 14, 2, 3, 7; (B) 1, 6, 14, 2, 6, 7; (C) 2, 10, 14, 2, 10, 7; (D) 2, 12, 28, 4, 12, 14arrow_forward12. Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: b. FeO(s) + CO(g) ⟶⟶ Fe(s) + CO2(g) ΔH = __________ 3Fe2O3(s) +CO(g) ⟶⟶ 2Fe3O4 +CO2 (g) ΔH = -68.26 kJ Eq 1 Fe2O3(s) + 3CO(g) ⟶⟶ 2Fe(s) + 3 CO2(g) ΔH = -23.44 kJ Eq 2 Fe3O4(s) + CO(g) ⟶⟶ 3FeO(s) + CO2(g) ΔH = +21.79 kJ Eq 3arrow_forwardHow is this equation derived? from pre-existing michaelis menten kinetics assumptions?arrow_forward
- 5arrow_forwardAG°xn=-28.6 kJ Given the following equation, H2O(g) + CO(g) → H2(g) + CO2(g) Calculate AG°n for the following reaction. -> 8 H2O(g) + 8 CO(g) 8H2(g) + 8 CO2(g) a -71.5 kJ b -3.57 kJ C +3.57 kJ d +228.8 kJ e -228.8 kJ O O O O Oarrow_forwardA patient presents to the emergency department in a critical condition. The patient’s blood was tested and the concentration of carbonic acid (H2CO3) was found to be 4.0×10-4 M and bicarbonate ion (HCO3-) 3.062×10-3 M. Ka = 4.3 × 10-7 H2CO3(aq) ⇌ HCO3-(aq) + H+(aq) Show all calculations including equation(s) used. Calculate the pH of the patients’ blood. Based on your answer would you say the patient is suffering from Acidosis or Alkalosis?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON