
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
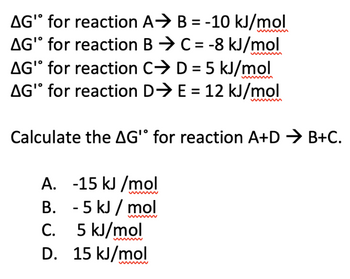
Transcribed Image Text:AG" for reaction
AG" for reaction
A⇒ B=-10 kJ/mol
BC = -8 kJ/mol
AG' for reaction
CD = 5 kJ/mol
AG" for reaction D⇒ E= 12 kJ/mol
Calculate the AG' for reaction A+D → B+C.
A. -15 kJ/mol
B.
- 5 kJ/mol
C.
5 kJ/mol
D. 15 kJ/mol

Transcribed Image Text:For reaction A ⇒B, AG¹° = -22 kJ/mol
For reaction BC, AG" = 0 kJ/mol
For reaction C ⇒ D, AG" = 31 kJ/mol
Which reaction has the largest K'eq?
A. A B
B. BA
C. B C
D. CI
E. D➜ C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- How is this equation derived? from pre-existing michaelis menten kinetics assumptions?arrow_forward1. Nestle Bearbrand is a famous milk. Therefore, many people consume such milk. At the time of drinking the milk, it feels sweetness in the milk. A. What are the source compounds of such sweetness? Write down the structure formula! B. How much does the compound weigh in 1 can of milk size 189mLc. How much energy (in the form of ATP) is produced from carbohydrates in the milk?d. Why is there Natrium in the milk? # To answer this question, please see the composition of milk with the details i given below ( or you can see the picture attched )Composition: Cow MilkThe dose of 1 can of 189 ml has; a total energy of 120 kcal, energy from fat 60 kcal . Total fat 7 g saturated fat 5.0 g Cholesterol 25 mgProtein 6 gCarbodirate Total 9 gand Natrium 115 mg# Conversion NADH and FADH2 = 2.5 and 1.5 ATP( Please write the answer on the paper thank you )arrow_forwardAG°xn=-28.6 kJ Given the following equation, H2O(g) + CO(g) → H2(g) + CO2(g) Calculate AG°n for the following reaction. -> 8 H2O(g) + 8 CO(g) 8H2(g) + 8 CO2(g) a -71.5 kJ b -3.57 kJ C +3.57 kJ d +228.8 kJ e -228.8 kJ O O O O Oarrow_forward
- A scientist extracted compounds from apple peel and separated them using gel exclusion chromatography. Indicate which would be the expected order of elution from first eluted to last.arrow_forwardDescribe reaction between AP and PNPP and how is used to determine the kinetic parameters of AP. i. İndicate the value for the activity of AP(which is 3.08) What does it mean? Is your enzyme active or not? What does it say about the purity of your enzyme? ii. How the purification process affects the activity?arrow_forwardCombining 0.304 mol Fe2O30.304 mol Fe2O3 with excess carbon produced 11.6 g Fe.11.6 g Fe. Fe2O3+3C⟶2Fe+3COFe2O3+3C⟶2Fe+3CO What is the actual yield of iron in moles? What is the theoretical yield of iron in moles? What is the percent yield?arrow_forward
- The most important factor guaranteeing a biochemical change is a. A positive ∆G b. Appropriate temperature c. A negative ∆S d. A negative ∆Garrow_forwardNaOH.2L X.02mol/L = .004 mol, H3PO4.8L X.15 mol/L = .12 mol H₂PO4 HPO4² + H+ .108 mol start A. If 200 mls of 0.02 M NaoH is added to 800 mls of 0.15 M phosphoric acid buffer at pH 8.2, what is the resultant pH? (pK1 = 2.1, Pk2=7.2, pk3=12.2) add .004 mol OH- end .012mol -.004 .008 mol pH= pka + log [A-]/[HA] = 7.2 + log.112/.008 +.004 .112mol pH=8.35 B. If the same amount of NaOH in part A is added to 800 mls of water, what is the resulting pH? .004mol/1L =.004 [OH-] pOH = 2.4, pH -11.6arrow_forwardPredict the approximate ratio peaks in the mass spectrum of 1,2-Dichloroethane at m/z values 98, 100, and 102.arrow_forward
- You are evaluating the kinetics of an enzyme catalyzed reaction containing 5.5 μM total enzyme and 11.2 μM substrate. At this substrate concentration, you determine that the Vo = 88.6 μmol mL-¹. s-¹. If the Vmax 833.3 mM s the KM is: . == " 30.9 μΜ 10.4 μΜ Ο 124.6 μΜ 234 μΜ 94.5 μMarrow_forwardUse the following balanced equation for problems 1–5. Molar masses are given below. 2 As 3 H2 2 AsH3 150. kcal + + molar masses 74.92 g 2.02 g 77.95 g How many moles of hydrogen are required to react with 25.00 moles of arsenic, As ? A. 75.00 mol B. 25.00 mol C. 16.67 mol D. 37.50 molarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON