
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
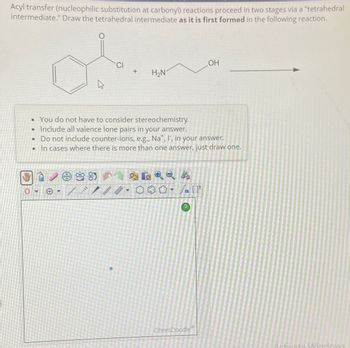
Transcribed Image Text:Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral
intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction.
0
OH
CI
+
H₂N
• You do not have to consider stereochemistry.
• Include all valence lone pairs in your answer.
• Do not include counter-ions, e.g., Na+, I, in your answer.
In cases where there is more than one answer, just draw one.
A
ChemDoodle
Activate Windows
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. You do not have to consider stereochemistry. Include all valence lone pairs in your answer. Do not include counter-ions, e.g., Na+, I-, in your answer. In cases where there is more than one answer, just draw one.arrow_forwardAcyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. You do not have to consider stereochemistry. Include all valence lone pairs in your answer. Do not include counter-ions, e.g., Na+, I-, in your answer. In cases where there is more than one answer, just draw one.arrow_forwardAcyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. H3C NH₂ HCI/H₂O reflux • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na+, I, in your answer. • In cases where there is more than one answer, just draw one.arrow_forward
- Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forwardStep 4a: Classify step. The target product is present, but the reaction is not over. The ethoxide ion has been regenerated. :0: :0: What is the key process in the next step of the mechanism? proton transfer formation of a o bond between nucleophile and electrophile loss of a leaving group carbocation rearrangement (e.g., methyl or hydride shift)arrow_forward1arrow_forward
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. H3C HCI / H2O `NH2 reflux • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na", I, in your answer. • In cases where there is more than one answer, just draw one. opy aste Previous Nextarrow_forwardFor the given Sy2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group. Include wedge-and-dash bonds and draw hydrogen on a stereocenter. :CEN Organic product Inorganic product + Draw the organic product. Draw the inorganic product. Incorrectarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the missing intermediates and product formed in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts.arrow_forward
- Use the dropdown menu to indicate whether the rate of the reaction shown below will increase, decrease, or remain the same when the reaction conditions are changed to X or to Y or to Z (see below). NaN3 'N3 Br CH3CN X: Change the leaving group from Br to CI Y: Increase the concentration of haloalkane Z: Increase the concentration of NaN3 X: choose your answer.. ^ Y: choose your answer... V Z: choose your answer... choose your answer... Remain the same Nex Decrease Increasearrow_forwardAcyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. H3C. ... NH2 HCI/H₂O reflux • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na+, I, in your answer. • In cases where there is more than one answer, just draw one. + 4 n [ ?arrow_forwardDraw the structure of the organic product formed when the given compounds undergo the three-step reaction sequence indicated. Select Draw Rings More Erase C Br 1. NaOC,H5. C,H5OH 2. NaOH, H2O 3. H3O*, heatarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning