
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Density Lab (Need help plotting this graph)
How to plot this graph from the data sheet (see picture attached) with mass of solution (on y-axis) vs volume of solution (on x-axis) for the 0%, 10%, 20%, 30%, 40%, 50%, 60% sucrose solutions so that it is all on one graph? There should be seven sets of data with seven best-fit lines on one graph. The y-intercept of the best-fit line is set to 0. Please include how to setup the data in excel spreadsheet to plot the graph.
Thank you
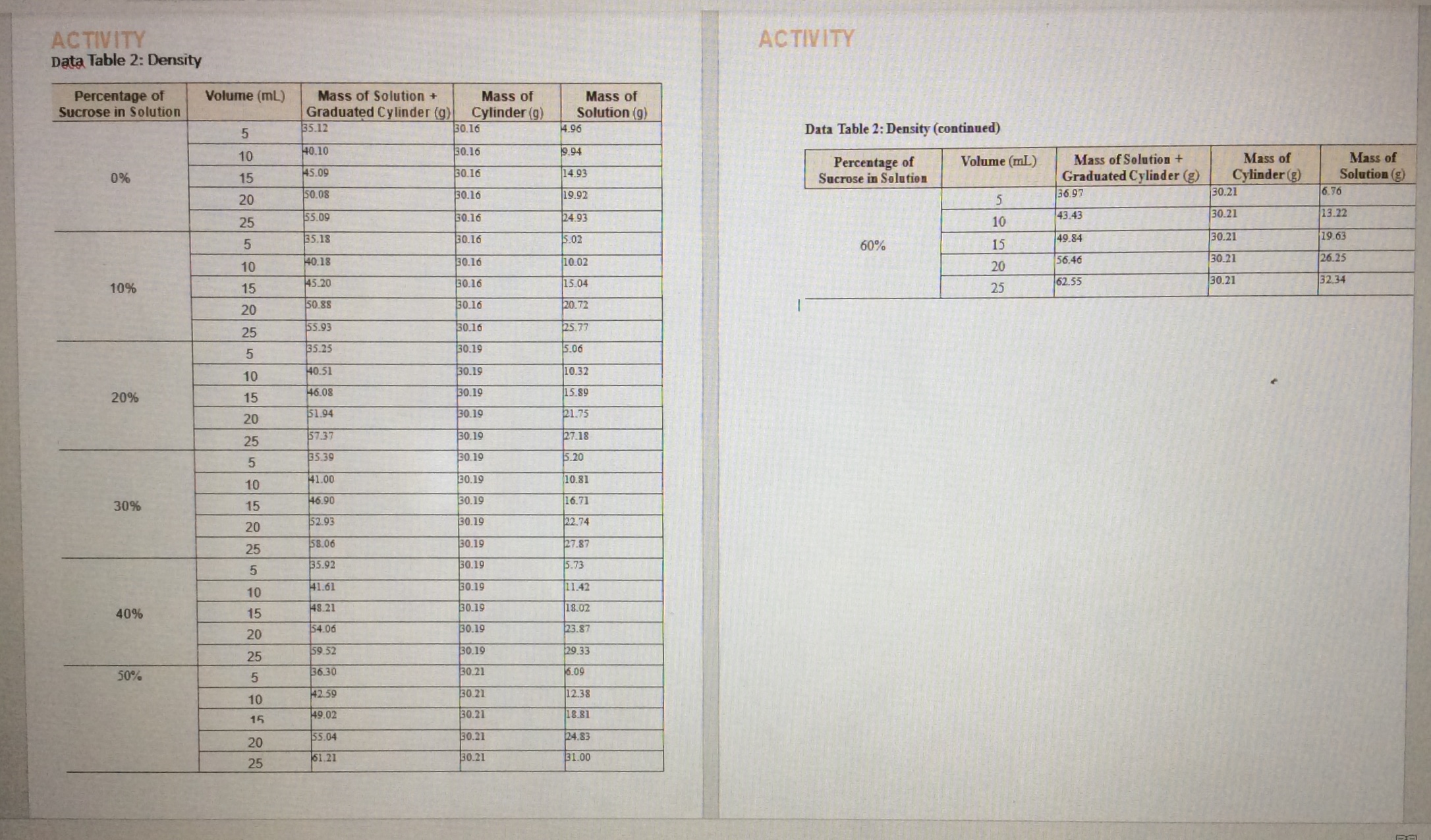
Transcribed Image Text:ACTIVITY
Data Table 2: Density
ACTIVITY
Percentage of Volume (mL) Mass of SolutionMass of
Sucrose in Solution
Mass of
Graduated Cylinder (g) Cylinder(g) Solution (g
5.12
Data Table 2: Density (continued)
0.16
0.16
94
Percentage ofVolume (mL) M
Sucrose in Solution
f tion +
Mass of
Mass of
5.09
0.16
14.93
Graduated Cylinder(
0%
6.76
30.21
0.08
36.97
0.16
19.92
13.22
30.21
43.43
0.16
4.93
5.09
10
25
9.63
30.21
5.18
49.84
0.16
15
60%
30.21
26.25
0.16
5646
0.02
30.21
32.34
62.55
5.20
15.04
0.16
25
10%
0.16
20
5.93
0.16
0.19
5.25
5
0.19
10.32
15.89
20%
1.75
1.94
0.19
7.18
7.37
5.39
20
1.00
0.19
10.81
0.19
16.71
30%
2.93
8.06
7.87
5.92
1.61
0.19
8.21
18.02
0.19
40%
06
9.52
6.30
21
50%
5
2.59
0.21
2.38
0.21
8.81
9.02
0.21
0.21
1.21
1.00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Based off information from the attachment, please help with #9 on second attachmentarrow_forwardarch F2 # 3 E D 1. Plot density versus concentration for the five reference solutions on a graph. The concentration is the independent variable (x-axis), and the density is the dependent variable (y-axis). Use a spreadsheet program or ruler to draw a "best-fit" straight line through the data points. II 2. Use the graph to estimate the unknown sugar concentration in each beverage. To do this, locate the point on the y-axis that corresponds to the density of the beverage. Follow that point on the y-axis horizontally to where it meets the best-fit straight line. Read down vertically from this point on the best-fit line to the x-axis to estimate the percent concentration of sugar in the beverage. Construct a Results Table and record the density of each beverage and its estimated percent sugar concentration. F3 LA $ 4 R LL Beverage Coke Sprite O E F4 % 5 T G F5 7 ^ 6 Density, g/mL ( CHM 101L-Survey of Chemistry | Laboratory Y F6 H S PrtScn & 7 U F7 Percent, %estimated Home 8 F8 9 JK Rain...…arrow_forwardHow would I find the last column of the table?arrow_forward
- A 1mL solution contains a 20X diluted sample. How do you make a spike solution that contains 100uL of the standard you will compare to the sample and 900uL of the diluting liquid? If you have a spike solution, do you still need to make a range of standard solutions? Please show detail, thank you.arrow_forwardA dilute aqueous solution containing 1 ppm of solute has a density of 1.00 g/ mL. Express the concentration of solute in g/L, g/mL, and mg/ L. (Show work/calculations)arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Please fill in blanks as shown on problem!!arrow_forwardAn aqueous solution of chromium(III) sulfate,Cr (SO4)3 , contains 8.67 grams of chromium(III) sulfate and 17.6 grams of water. What is the weight/weight percent of chromium(III) sulfate in the solution? Weight/weight percent =_____ %arrow_forwardSHOW YOUR WORK! Use conversion factors to determine the answers to the Following questions. Do not forget sig figs & units. #47arrow_forward
- Concentrated Standard Solution: 3.0168 weighed mass (NH4)2SO4-FeSO4-6H2O g in L of solution 99.70 purity (NH4)2SO4-FeSO4-6H2O Volume 2.000L Diluted Standard Solution mass (NH4)2SO4-FESO+-6H2O per L of solution mg/L(or ppm)** Fe concentration (using % purity) ppmarrow_forward||| MEASUREMENT AND MATTER One step dosage calculations An elderly patient with chronic osteoarthritis experiencing a flare-up is to receive 0.9 mg of dexamethasone by injection. The drug vial is labeled "dexamethasone sodium phosphate 4.0 mg/mL." How many mL of the solution in the vial should be drawn up in a syringe for administration to the patient? Round your answer to the nearest 0.01 mL. mL Explanation B Check X Q Search 0/3 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility wwwpet @arrow_forward5. The mineral analysis below was reported for a water sample. Calcium 90 Chloride 120 Magnesium 30 Sulfate 170 Iron (Fe**) 5 Bicarbonate 165 Sodium 10 pH 7.5 units Potassium 6. Note: All reported as "mg/L as the ion" unless stated otherwise. Draw a bar chart that includes all the ions listed above. (Remember to convert to appropriate units) b. What is the total hardness of the water using ALL multivalent cations in mg/L as а. CACO3? c. What is the total hardness of the water using the predominant polyvalent cations in mg/L as CaCO3? d. What is the percent error in using only the predominant polyvalent cations? e. What is the carbonate hardness of the water in mg/L as CaCO3? f. What is the non-carbonate hardness of the water in mg/L as CACO3?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY