
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
During lab, a student used a Mohr pipet to add the following solutions into a 25 mL volumetric flask. They calculated the final volumes added, which are recorded below. The student then followed the directions in the manual to make the stock solution.
Volumes Used to Create Stock Solution
| Volume 0.200 M Fe(NO3)3 (mL) | Volume 0.00200 M KSCN (mL) | |
|---|---|---|
| Stock Solution | 9.60 mL | 2.55 mL |
Using the stock solution above, the student made additional dilutions, with the final volumes below. Calculate the concentration of iron(III) thiocyanate ion in Standard 2. For grading purposes, report your answer in mM with four places after the decimal.
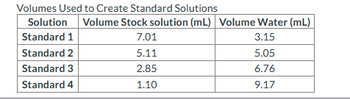
Transcribed Image Text:Volumes Used to Create Standard Solutions
Solution Volume Stock solution (mL) Volume Water (mL)
Standard 1
3.15
5.05
6.76
9.17
Standard 2
Standard 3
Standard 4
7.01
5.11
2.85
1.10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5.00 mL of 5.09 M Fe(NO3)3 is combined with 1.00 mL of 0.50 M HCIO4 and 4.00 mL of 2.00 x 10-2M KSCN. What is the concentration of Fe3+ in the solution after the other reactants are added? Enter your answer with two decimal places. Example: 1.32 Do not include units in your answer. 2.55 A Moving to another question will save this response. MacBook Air F7 F8 F2 F3 000 F4 F5 F6 $ % & 6. 8 * 00 5 %#3arrow_forwardConsider the colored precipitates you produced and the cations in those reactions. Identify the colorful cations and describe their location on the periodic table. What do they have in common? Select the statement that best answers the question They are from the same row on the periodic table They are from the same column on the periodic table They are all nonmetals They are transition metals They are all alkali metalsarrow_forwardA solution is initially 0.10 M in Mg2*(aq) and 0.10 M in Fe2* Mg(OH)2 first precipitates? Ksp (Mg(OH)2) = 6.0 x 10-10; Ksp(Fe(OH)2) = 7.9 x 10-16 your answer in E-notation, rounded to 2 significant digits. *(aq). A solution of NaOH is slowly added. What is the concentration of Fe2+ when %3D Providearrow_forward
- - Part C Write a balanced ionic equation for this acid-base reaction: Ca(OH)2 (aq) + 2CH3CO₂H(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer. ΑΣΦ A chemical reaction does not occur for this question. Submit Request Answer Part D ? Write net ionic equation for this acid-base reaction: Ca(OH)2 (aq) + 2CH3CO₂H(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forwardIf 21 mL of 3.2 x 10–5 M magnesium chloride and 15 mL of 1.5 x 10–4 M sodium fluoride are mixed, what are the concentrations of magnesium ion, chloride ion, sodium ion, and fluoride ion after mixing? Some choices may be used more than once, and keep an extra sig fig. Question 9 options: chloride ion sodium ion magnesium ion fluoride ion 1. 1.87 x 10–5 M 2. 3.73 x 10–5 M 3. 6.25 x 10–5 M 4. 1.25 x 10–4 M 5. 1.50 x 10–4 M 6. 3.20 x 10–5 M 7. 1.50 x 10–4 M Answer and Explainarrow_forwardPlease use the given info to find x in the equation (Hint: y = 0.0472x+0.9895).arrow_forward
- 2- A sample of an acid 2.4 g dissolved in 100 ml. water. This sample was titrated with 40 ml sodium hydroxide (0.10 N). Find out the molecular mass of the acid knowing that it is mono-equivalent.arrow_forwardConsider the neutralization reaction 2 HNO, (aq)+Ba(OH), (aq) 2 H, O(1)+Ba(NO,),(aq) A 0.115 L sample of an unknown HNO, solution required 36.7 mL of 0.250 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration: M * TOOLS x10arrow_forwardEqual volumes of Solutions 2 and 3 were mixed in the lab. Suggest a measurement that could be made in the lab to provide evidence that a chemical reaction occurred when Solutions 2 and 3 were mixed.arrow_forward
- Part B A chemist has a bottle containing 0.57 M CuCl2. How many milliliters of this solution should the chemist use to add 2.0 grams of CuCl2 to her reaction? 3.5 mL 26 mL 13 mL 0.015 mLarrow_forwardJust number 3 please!arrow_forwardYou are asked to prepare a 1.000 L solution of 4.5 M you commit a user error while preparing this solution. Assumed volume Volumetric error Preparation details Added water 2.0 cm above the line, which corresponds to 8.2 mL (0.0082 L) additional solution volume C6H12O6 (glucose; molar mass = 180.16 g/mol) in a lab by dissolving 811.0 g of glucose in water. Consider the following two scenarios in whic Prepared in a beaker Prepared in a volumetric flask 1.000 L You add the glucose to a volumetric flask and then add water until it dissolves. The water bottle you are using has a worn tip, and you inadvertently add too much water such that the meniscus is above the line. The diameter of the neck of the volumetric flask is 2.29 cm. 811.0 g glucose Concentration: You decide to evaluate and compare the errors you made while preparing the solutions using the different methods. Calculate the actual concentrations of the intended 4.5 M glucose solutions prepared by each method based on their…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY