
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
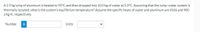
Transcribed Image Text:A2.9 kg lump of aluminum is heated to 95°C and then dropped into 10.0 kg of water at 5.0°C. Assuming that the lump-water system is
thermally isolated, what is the system's equilibrium temperature? Assume the specific heats of water and aluminum are 4186 and 900
J/kg-K, respectively.
Number
i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- (a) Estimate the specific heat capacity of sodium from the Law of Dulong and Petit. The molar mass of sodium is 23.0 g/mol. (b) What is the percent error of your estimate from the known value, 1230 J/kg ? `arrow_forwardSuppose 26.0 g of neon gas are stored in a tank at a temperature of 152C. (a) What is the temperature of the gas on the Kelvin scale? (See Section 10.2.) (b) How many moles of gas are in the tank? (See Section 10.4.) (c) What is the internal energy of the gas? (See Section 10.5.)arrow_forward(a) What is the gauge pressure in a 25.0 cc car tire containing 3.60 mol of gas in a 30.0-L volume? (b) What will its gauge pressure be if you add 1.00 L of gas originally at atmospheric pressure and 25.0 ? Assume the temperature remains at 25.0 cc and the volume remains constant.arrow_forward
- A 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? Find (c) Q, (d) Eint, and (e) W for the gas during this process.arrow_forwardA tank contains 111.0 g chlorine gas l2), which is at temperature 82.0 and absolute pressure 5.70105 Pa. The temperature of the air outside the tank is 20.0 . The molar mass of Cl2 is 70.9 g/mol. (a) What is the volume of the tank? (b) What is the internal energy of the gas? (c) What is the work done by the gas if the temperature and pressure inside the tank drop to 31.0 and 3.80105 Pa, respectively, due to a leak?arrow_forwardIn 1986, a gargantuan iceberg broke away from the Ross Ice Shelf in Antarctica. It was approximately a rectangle 160 km long, 40.0 km wide, and 250 m thick. (a) What is the mass of this iceberg, given that the density of ice is 917kg/m3 ? (b) How much heat transfer (in joules) is needed to melt it? (c) How many years would it take sunlight alone to melt ice this thick, if the ice absorbs an average of 100W/m2, 12.00 h per day?arrow_forward
- (a) It is difficult to extinguish a fire on a crude oil tanker, because each liter of crude oil releases 2.80107 J of energy when burned. To illustrate this difficulty, calculate the number of liters of water that must be expended to absorb the energy released by burning 1.00 L of crude oil, if the water's temperature rises from 20.0 C to 100 C, it boils, and the resulting steam's temperature rises to 300 C at constant pressure. (b) Discuss additional complications caused by the fact that crude oil is less dense than water.arrow_forwardWhat is the average mechanical energy of the atoms of an ideal monatomic gas at 300 K?arrow_forward(a) Calculate the rate of heat transfer by radiation from a car radiator at 110C into a 50.0C environment, if the radiator has an emissivity of 0.750 and a 1.20m2 surface area. (b) Is this a significant fraction of the heat transfer by an automobile engine? To answer this, assume a horsepower of 200 hp (1.5 kW) and the efficiency of automobile engines as 25%.arrow_forward
- (a) Calculate the work done by the gas along the closed path shown below. The curved section between R and S is semicircular. (b) If the process is carried out in the opposite direction, what is the work done by the gas?arrow_forwardOne process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of about 14,0 mol/m3 and a temperature of about 60 . (a) Is CO2 a solid, liquid, gas, or supercritical fluid under those conditions? (b) The van der Waals constants for carbon dioxide are a=0.3658 Pa m6/mol2 and b=4.286105 m3/mol. Using the van der Waals equation, estimate pressure of CO2 at that temperature and density. `arrow_forwardIn an insulated vessel, 250 g of ice at 0C is added to 600 g of water at 18.0C. (a) What is the final temperature of the system? (b) How much ice remains when the system reaches equilibrium?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning