
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
4.0 moles of krypton gas are in a 0.20 m3 container. The pressure is 1.663 × 105 Pa.
a. What is the temperature (to the nearest K)?
The volume contracts to 0.10 m3. The pressure is held constant.
b. How much work was done by the gas during the volume contraction?
c. What is the temperature after the volume contraction (to the nearest K)?
d. What was the change in thermal energy?
e. What was the heat flow? Express in units of J, positive = into gas, negative = out of gas.
Expert Solution
arrow_forward
Step 1
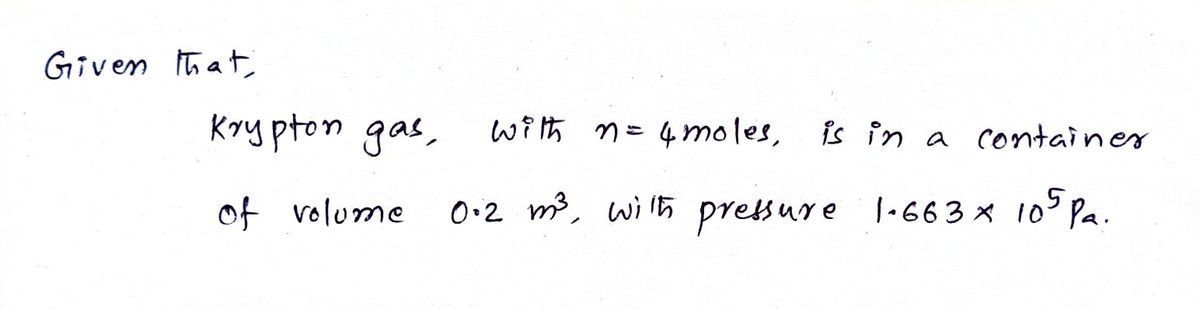
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements is not true according to kinetic theory? a. The molecules in an ideal gas undergo elastic collisions. b. The molecules in an ideal gas only interact during collisions. c. Electrical attractions and repulsions between the molecules must be accounted for in the total energy of the ideal gas. d. The average energy of a molecule in an ideal gas is evenly distributed between the different degrees of freedom of the molecule. e. The energy of an ideal gas only depends on the kinetic energy of the gas.arrow_forwardA container of nitrogen molecules is at a temperature of 37.0°C. What is the mass of a nitrogen molecule in atomic mass units? [Note: nitrogen is a diatomic gas.] а. b. What is the mass of a nitrogen molecule in kilograms? C. What is the average translational kinetic energy of the nitrogen molecules? d. What is the rms speed of the nitrogen molecules? е. What is the average rotational energy of a nitrogen molecule?arrow_forwardThe heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) Figure p (kPa) 600 400 200- 0 0 0.025 0.050 V (m³) Part A Determine T₁, T₂, and T₁. Enter your answers numerically separated by commas. Express your answer using two significant figures. T₁ T₂. T₂ = 600,1800,1200 K Submit Previous Answers ✓ Correct Part B Determine AEth. W. and Q for 1-2. Enter your answers numerically separated by commas. Express your answer using two significant figures. AEth. Ws. Q = 3.0x104.1.3x104.4.3x104 J Submit ✓ Correct ▾ Part C Previous Answers Determine AEth. W. and Q for 2-3. Enter your answers numerically separated by commas. Express your answer using two significant figures. AEth. Ws. Q= Submit ▾ Part D ΠΑΣΦ Request Answer I ? Jarrow_forward
- Estilos Edición P5. A rigid container contains water vapor at 250°C and an unknown pressure. When the container cools to 150°C, the vapor begins to condense. Estimate the initial pressure in the container. Plot the thermodynamic process on a phase diagram. Answer: 600 kPa.arrow_forwardA 2100 cm^3 container holds 0.15 mol of helium gas at 330C. 1. How much work must be done to compress the gas to 1400cm^3 at constant pressure? 2. How much work must be done to compress the gas to 1400cm^3 at constant temperature?arrow_forward-70 J of work are done on the gas in the process shown in (Figure 1). Figure p (kPa) P₁ 0+ 0 100 200 300 < 1 of 1 V (cm³) Part A What is p₁ in kPa? Express your answer in kilopascals. P1 = Submit VE ΑΣΦ Provide Feedback Request Answer ***** ? kPaarrow_forward
- A cube 19 cm on each side contains 4.0 g of helium at 20*C. 1000 J of heat energy are transferred to this gas. A. What is the final pressure if the process is at constant volume? B. What is the final volume if the process is at constant pressure?arrow_forwardA cylinder containing 5.00 mol of nitrogen gas is at room temperature (300 K). If the pressure of the gas is held constant and the gas is heated, how much energy must be transferred by heat to the gas to increase its temperature by 100 K? Select one: O a. 3.24 x 104 J O b. 1.46 x 104 J O c. 2.15 x 104J d. 0.94 x 104 J O e. 1.04 x 104 J Oarrow_forward0.0042 mol of gas undergoes the process shown in (Figure 1). You may want to review (Pages 503 - 508). igure p (atm) 3 2 1 0- 0 100 2 200 300 1 of 1 V (cm³) Part B What is the initial temperature in °C? Express your answer using two significant figures. T₁ = Submit Part C Tf = What is the final temperature in °C? Express your answer using two significant figures. Submit VG ΑΣΦ Provide Feedback Request Answer ΑΣΦ Request Answer ? 11? °Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON