
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
A.Provide the name for the D-monosaccharide “1”
in the above image. You may ignore the alpha
or beta anomer label.
B. Provide the name for the D-monosaccharide “2”
in the above image. You may ignore the alpha or
beta anomer label.
C.Provide the name for the D-monosaccharide “3”
in the above image. You may ignore the alpha or
beta anomer label.
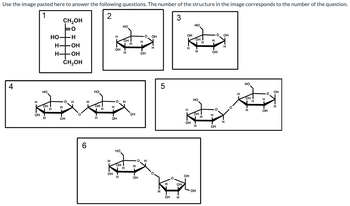
Transcribed Image Text:The image contains six diagrams of chemical structures, which appear to represent different forms of carbohydrates. Below is a transcription and detailed description for each numbered structure:
1. **Structure 1:**
- This is a linear structure showing D-glucose in its Fischer projection form. The formula depicted is CH₂OH-CHOH-HO-CHOH-H-C=O-CH₂OH.
- At the center is an aldehyde group (CO), with hydroxyl groups (OH) attached to the carbon chain.
2. **Structure 2:**
- This structure is a cyclic form of glucose, called the Haworth projection. It shows the glucose molecule in its beta-D-glucose form.
- The hydroxyl groups are oriented above the plane at C-1 and below at C-2, C-3, and above at C-4.
3. **Structure 3:**
- This is another cyclic form of glucose, also in the Haworth projection, representing alpha-D-glucose.
- The hydroxyl group at C-1 is below the plane, while hydroxyl groups at C-2 and C-4 are above.
4. **Structure 4:**
- This diagram shows a disaccharide formed by two glucose units, appearing to represent maltose.
- The linkage is an alpha-1,4-glycosidic bond, capturing the bond between the C-1 of one glucose and C-4 of the other.
5. **Structure 5:**
- This structure is a polysaccharide chain, likely showcasing a segment of amylose or starch.
- The glucose units are connected through alpha-1,4-glycosidic bonds, creating a helical formation.
6. **Structure 6:**
- This diagram displays a branched polysaccharide, probably glycogen or amylopectin.
- The connection includes both alpha-1,4- and alpha-1,6-glycosidic linkages, with the latter indicating branching.
These representations illustrate various structural forms of glucose and its polymers, highlighting key differences in stereochemistry and bonding that affect their biological function and properties.
Expert Solution
arrow_forward
Step 1
There are four classes of biological macromolecule: nucleic acids, proteins, lipids and carbohydrates.
The carbohydrates can be classified as monosaccharides, disaccharides and polysaccharides. Of these the monosaccharides are known as simple sugar having a general chemical formula C6H12O6
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Select all that applyarrow_forwardNonearrow_forwardA) Refer to the figure below, Identify and explain the two types of reactions, and describe what are the importance of these reactions to our bodies. Reaction 1 H Monomer Reaction 2 H H OH + H H H₂O H H₂O OH + H H OH OH + H H₂O H₂O OH OH + H OH OH OH OH B) What are the main chemical interactions that determine and maintain the quaternary structure of proteins. Also, what are the conditions that can alter these interactions? [arrow_forward
- a. Why is it important to eat food containing antioxidants? Write at leasttwo reactions to prove the answer. b. Write in four ways the following nucleotide sequence: ATGCA. c. Explain i. Hoogsteen pairing & ii. Hyperchromic effectiii. Epimers Iv. Mutarotaton v. Aldose vi. Anomers vii. Mutarotationarrow_forwardEstimate the PI of the given amino acid? Assume that the amino acid contains a single carboxylic acid group on the side chain.arrow_forwardIn 3 sentences, explain this. Discuss the difference between an ⍺-D-glucose and a β-D-glucose. How can the structure of a monosaccharide affect its bioactivity?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON