
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please explain how to fill in table given provided information thanks
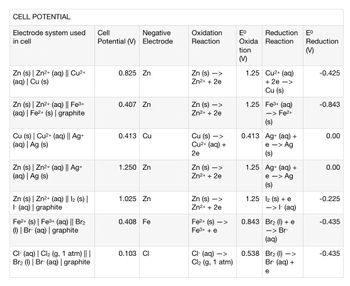
Transcribed Image Text:CELL POTENTIAL
Electrode system used
in cell
Zn (s) | Zn²+ (aq) || Cu²+
(aq) | Cu (s)
Zn (s) | Zn²+ (aq) || Fe³+
(aq) | Fe²+ (s) | graphite
Cu (s) | Cu²+ (aq) || Ag+
(aq) | Ag (s)
Zn (s) | Zn²+ (aq) || Ag+
(aq) | Ag (s)
Zn (s) | Zn²+ (aq) || 12 (s) |
1- (aq) | graphite
Fe2+ (s) | Fe³+ (aq) || Br₂
(1) | Br- (aq) | graphite
CI- (aq) | Cl₂ (g, 1 atm) || |
Br2 (1) Br- (aq) | graphite
Cell
Negative
Potential (V) Electrode
0.825 Zn
0.407 Zn
0.413 Cu
1.250 Zn
1.025 Zn
0.408 Fe
0.103 CI
Oxidation
Reaction
Zn (s)->
Zn²+ + 2e
Zn (s) ->
Zn²+ + 2e
Cu (s) ->
Cu²+ (aq) +
2e
Zn (s)->
Zn²+ + 2e
->
Zn (s)
Zn²+ + 2e
Fe²+ (s)->
Fe³+ + e
Cl- (aq) ->
Cl₂ (g, 1 atm)
Eº Reduction Eº
Oxida Reaction
tion
(M)
1.25
Cu²+ (aq)
+ 2e ->
Cu (s)
1.25 Fe³+ (aq)
-> Fe²+
(s)
0.413 Ag+ (aq) +
e -> Ag
(s)
1.25 Ag+ (aq) +
e
> Ag
(s)
1.25 12 (s) + e
0.538
-> 1- (aq)
0.843 Br2 (1) + e
-> Br-
(aq)
Br2 (1) –>
Br- (aq) +
e
Reduction
(M)
-0.425
-0.843
0.00
0.00
-0.225
-0.435
-0.435

Transcribed Image Text:264 Experiment 32 Voltaic Cell Measurements
A.2. Table of Electrode Potentials
In Table A.1, you should have a value for Ed or Exid for each of the electrode systems you have studied.
Remembering that for any electrode system, Ered =-E0
Eoxid, you can find the value for Ered for each system. List
those potentials in the left column of the table below, in order of decreasing (more negative) value.
Ereduction
Ag+,Ag
= 0.00 V)
Electrode reaction
in reduction
(Eº
=
Ereduction
H+,H₂
= 0.00 V)
The electrode potentials you have determined are based on setting Egt.Ag 0.00 V. The usual convention in
electrochemistry is to set EH¹,H₂ = 0.00 volts, under which conditions E
Ag+,Ag red
= +0.80 V. Convert from
one base to the other by adding 0.80 volts to each of the electrode potentials in the first column, and enter
these values in the third column of the table.
Why are the values of Ed on the two bases related to each other in such a simple way?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the possible classification of the unknown solution?arrow_forward20.00mL of 6M HCl is taken from 500mL bottle containing the acid and is transferred to 250mL volumetric flask and diluted to volume with water. What is the molarity of the new solution? Do not enter units with your answer.arrow_forwardMatch the following terms to their definitions: Aqueous solution, cation, centrifuge, decant, flame test, precipitate, qualitative analysis, supernate the systematic separation and identification of the chemical components in an unknown sample is known as ...? Group of answer choices Centrifuge Decant Supernate Flame Test Aqueous Solution Precipitate Qualitative Analysis Cationarrow_forward
- 0.1890 grams of an unknown organic acid containing 2 carboxylic groups was dissolved in about 50 mL of water in a 250 mL Erlenmeyer flask and titrated against 0.1000 M NaOH solution using bromothymol blue indicator. 34.62 mL of titrant was used to achieve end point. Calculate the molar mass of the unknown. Write answer with two decimal places only. No units please.arrow_forwardDATA AND CALCULATIONS Show all calculations neatly on an attached sheet. Trial 1 Trial 2 Trial 3 70.6849. 68.0749 2.61g 23.25ML O.65ML 22.6mL Mass of flask + vinegar Mass of empty flask Mass of vinegar used Final buret reading Initial buret reading Volume of NaOH used Moles of NaOH used 0.00226 mol Moles of açetic acid titrated Mass of acetic acid titrated Mass % acetic acid in vinegar 5.0%arrow_forwardSelect one for each boxarrow_forward
- 2.2 6. (1) Define pH in words. The strong acid HClag has a pH value of 1, use the following equation for a strong acid: Claa HClaa Hog + and convert the following expression to deduce the hydrogen ion concentration: pH = -log10 [H+] (ii) Use the above expression to deduce the pH of HCl (ag) given the concentration of the acid to be 4.5 mol/dm³ pH =arrow_forward1-Pentanol to 1-bromopentane Chemicals: - 60ml Conc. Sulfuric Acid - 100ml Saturated Sodium bicarbonate - 65ml 1-Pentanol - 78g sodium bromide - Distilled water - 58.42g 1-Bromopentane 1-Pentanol Sodium Bromide Sulfuric Acid 1-Bromopentane Formula C5H12O NaBr H2SO4 C5H11Br MW (g/mol) 88.15 102.894 98.078 151.04 Density (g/mL) 0.811 3.21 1.84 1.218 Boiling point (*C) 138 1,396 337 130 NaBr(aq) + H2SO4(aq) -> NaHSO4(aq) + HBr(aq) CH3(CH2)4OH(aq) + H+ Br- (aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4Br(aq) + H2O(aq) How do I calculate the percent yield and identify the limiting reagent?arrow_forwardHello, I hope you are doing well on this fine day. For the following quetion please read carefully the question and instruction. PLEASE ANSWER QUESTION IN 20 MINTUES NOT MORE PLEASE AND THANK YOU. If you do answer the question correctly and post it in the next 15 minutes, NO NEED TO SHOW THE WORK, I JUST WOULD LIKE THE CORRECT ANSWER AS SOON AS POSSIBLE. I will write a wonderful and generous feedback/review/rating about you. 2.077 g of salicylic acid is used in the reaction to synthesize ethyl salicylate, what is the minimum amount of ethanol in mL necessary to convert all of the salicylic acid to ethyl salicylate? The molecular weight of salicylic acid is 138.12 g/mol, the molecular weight of ethyl salicylate is 166.176 g/mol. The molecular weight of ethanol is 46.069 g/mol and its density is 0.789 g/mL.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY