
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please help fill in the rest of the table using the provided data. Show your work.
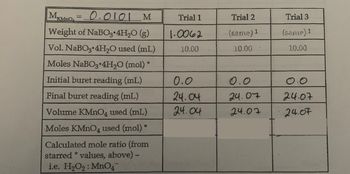
Transcribed Image Text:|MKMnO₂ = -0.0101 M
Weight of NaBO3 4H₂O(g)
Vol. NaBO3 4H₂O used (mL)
Moles NaBO3 4H₂O (mol)*
Initial buret reading (mL)
Final buret reading (mL)
Volume KMnO4 used (mL)
Moles KMnO4 used (mol)*
Calculated mole ratio (from
starred *values, above) -
i.e. H₂O₂: MnO4
Trial 1
1.0062
10.00
0.0
24.04
24.04
Trial 2
(same) 1
10.00
24.07
24.07
Trial 3
(same) 1
10.00
0.0
24.07
24.07
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Based on the previously asked question and it's data, please help fill in the rest of the table. Show your work. The molarity of KMnO4 is 0.0101M.
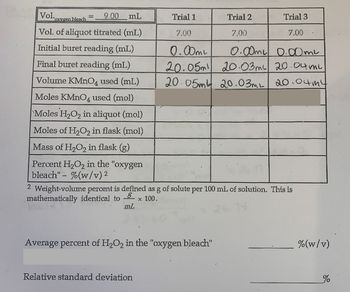
Transcribed Image Text:Vol.xver
9.00 mL
"Oxygen bleach
Vol. of aliquot titrated (mL)
Initial buret reading (mL)
Final buret reading (mL)
Volume KMnO4 used (mL)
Moles KMnO4 used (mol)
Moles H₂O₂ in aliquot (mol)
Moles of H₂O₂ in flask (mol)
Mass of H₂O₂ in flask (g)
Percent H₂O₂ in the "oxygen
bleach" - %(w/v) ²
2
mL
Trial 1
Trial 3
7.00
7.00
0.00ml
0.00ml 0.00mL
20.05m 20.03m² 20.04 mi
20.05m 20.03mL 20.04 my
2 Weight-volume percent is defined as g of solute per 100 mL of solution. This is
mathematically identical to
× 100.
Average percent of H₂O2 in the "oxygen bleach"
Relative standard deviation
Trial 2
7.00
%(w/v)
%
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Based on the previously asked question and it's data, please help fill in the rest of the table. Show your work. The molarity of KMnO4 is 0.0101M.

Transcribed Image Text:Vol.xver
9.00 mL
"Oxygen bleach
Vol. of aliquot titrated (mL)
Initial buret reading (mL)
Final buret reading (mL)
Volume KMnO4 used (mL)
Moles KMnO4 used (mol)
Moles H₂O₂ in aliquot (mol)
Moles of H₂O₂ in flask (mol)
Mass of H₂O₂ in flask (g)
Percent H₂O₂ in the "oxygen
bleach" - %(w/v) ²
2
mL
Trial 1
Trial 3
7.00
7.00
0.00ml
0.00ml 0.00mL
20.05m 20.03m² 20.04 mi
20.05m 20.03mL 20.04 my
2 Weight-volume percent is defined as g of solute per 100 mL of solution. This is
mathematically identical to
× 100.
Average percent of H₂O2 in the "oxygen bleach"
Relative standard deviation
Trial 2
7.00
%(w/v)
%
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H. H H-O H-O H. H 0-1 O H 0-H H O-H H 1 (CH) Н. H-O oi H- H-O H H -H H-O 0-H 22. The particle diagrams above show the dissolution of an unknown solid in water. Which of the following is best illustrated by the particle diagrams?arrow_forwardWith explanation of why the chosen answer is wrong and why the new one is correct! Thank you.arrow_forward2. Explain how suction filtration is carried out in the laboratory. Give the important points that must be observed in doing the process.arrow_forward
- Item 10 10 of 33 Complete I Review | Constants I Periodic Table Part A The compound MgCl2 is named dimagnesium chloride. magnesium chlorine. magnesium (II) chloride. magnesium dichloride. magnesium chloride.arrow_forwardPlease answer question number 1 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. Question 3: Calculate the molar mass of each of the following ionic Compounds: A. KMnO4 B. Ca3(PO4)2arrow_forwardЕ. NaCN Br Acetonearrow_forward
- In your own words, describe why metals are such good conductors of electricity. Be sure to include the definition of metallic bond in your answer. Answer in 2 to 3 complete sentences. I !!! H Normal A Enter your answer here BIUS √x 20 Txarrow_forwardWhat shift will this make? Shift left, shift right or no shift? How do you know?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY