
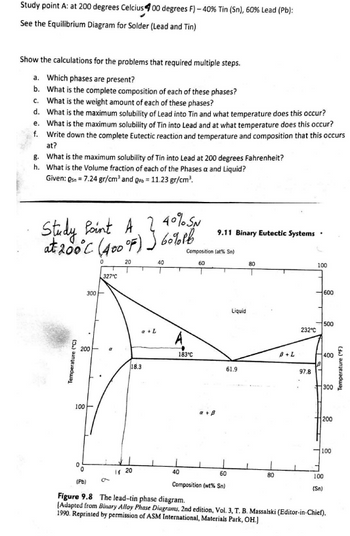
To determine the phase present at the point A () -40 % tin and 60 Pb. Also the complete composition of each phase and the weight composition of each phase at the given point is needed to be determined.
“Since you have posted a question with multiple sub-parts, we will solve the first three subparts for you. To get the remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.”
Step by stepSolved in 4 steps with 3 images

at 100C what is the maximum solubility (a) of Pb in Sn and (b) of Sn in Pb? The lead-tin phase diagram is shown in the animated figure
wt% Pb ?
wt% Sn ?
at 100C what is the maximum solubility (a) of Pb in Sn and (b) of Sn in Pb? The lead-tin phase diagram is shown in the animated figure
wt% Pb ?
wt% Sn ?
- 1. Which of the following is not a step in preparing a water sample container?a. All sample containers must be dark in colorb. The type of sample container and the level of cleaning required depend on the type of sample to be takenc. All sample containers must be thoroughly cleaned in the laboratory before sampling is carried outd. The number of containers prepared must always be in excess of what is needed, for quality assurance, quality control and reserves 2. The purpose of environmental sample analysis is..a. To determine the origin and concentration of chemicals in the environmentb. To determine the origin, concentration of chemicals and/or pollutants in the environmentc. To determine the concentration of a chemical in the environmentd. To determine the cause and concentration of pollutants in the environmentarrow_forward13. The following substance(s) does(do) not dissolve in water. A. cooking oil B. sugar C. salt D. vinegar E. alcoholarrow_forwardShow complete solution... Water has a specific heat of 4.18 J/goC. If 35.0 g of water at 98.8oC loses 4.94 kJ of heat, what is the final temperature of the water?arrow_forward
- 10. A 4 M solution of NaOH means a. 4 moles of NaOH in 1 L b. 4 grams of NaOH in 1L c. 4 moles of NaOH in 100 mls 11. What is a solvent? a. The portion of a mixture present in the largest quantity b. The portion of a mixture present in the smaller quantity 12. If we dissolve 116 grams of NaCl in one Liter of water, what is the Molarity of the solution? c. 58 M d. 1 M e. 2 M 13. We have 12 M HCl and need 4 L of 6 M HCl. How many Liters of 12 M HCl should we use? a. 3 L b. 4 L c. 2 Larrow_forwardCalculate the mass percent of solute in each solution. Calculate the mass percent of 3.97 g KCl dissolved in 42.6 g H2O. solute:? % Calculate the mass percent of 29.3 g KNO3 dissolved in 918 g H2O. solute:? %arrow_forward5.50 L of 13.3 M H 2 CO, the formaldehyde used to “fix” tissue samples 1.Number of moles2. Total mass of the solution3. Mass in grams (g) of a solutionarrow_forward
- how to separate a solid mixture of lithium bromide and barium carbonatearrow_forwardYou have 100. g of a solution that is 12% KCl by mass. How many grams of KCl are in the solution?arrow_forwardDetermine if crystalline sodium chloride and water are incompatible. If they are incompatible, state the hazardous conditions which would result from mixing the wastes.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





