
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
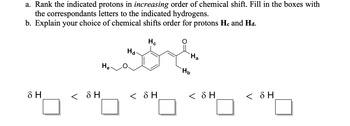
Transcribed Image Text:a. Rank the indicated protons in increasing order of chemical shift. Fill in the boxes with
the correspondants letters to the indicated hydrogens.
b. Explain your choice of chemical shifts order for protons He and Hd.
Hc
Ha
Ha
He
< SH
< δΗ
8 H
< SH
Hb
< SH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the following spectra and other information provided, identify the compounds, and all pertinent peaks are assigned in the spectra (correct compound, labeling proton spectrum, labeling carbon spectrum) Label the peaks on the spectrum and place the structure of the compound in the box on the right side of the spectrum.arrow_forwardCounterfeit drugs are a common problem in developing regions of the world. Oftentimes, counterfeit pills are made with compounds such as lactose. A lab technician has obtained the IR spectrum shown above for a sample reported to be citalopram, an antidepressant drug. Does the IR spectrum belong to citalopram or lactose? Explain your answer by describing what feature of the IR spectrum confirms your choice and describe what feature is missing from the IR spectrum for the other compound. A. citalopram B. lactosearrow_forward3. Protons A-E are identified on the spectrum below and correspond to the five unique protons in the molecule shown. (a) Place all the protons on the molecule and categorize them into Ha, Hb, etc. (b) Fill in the table for this molecule (see answer sheet). E الاس D N PPM B 72 Aarrow_forward
- Explain the relative chemical shifts of the benzene ring protons in Figurearrow_forwardTotal number of stereoisomers possible for the following compounds is HINT: a. 4 How many possible stereoisomers? How do we know how many stereoisomers are possible for a given structure? There is actually a straightforward way to figure this out. All we need to do is count the number of chiral centers and stereogenic alkene groups, the use this following rule: number of stereoisomeric forms = 2" ... where n = the number of chiral centers plus the number of stereogenic alkene groups b. 2 Consider for example a molecule with two chiral centers and one stereogenic alkene. By the rule stated above, we know right away that there must be eight possible stereoisomers. terminal alkene OH stereogenic alkene C. 8 OH d. 3 OH chiral center HO OH 3 2 = 8 T nonstereogenic alkenes (cannot be labelled E or Z) ←arrow_forwardA 300-MHz spectrometer records a proton that absorbs at a frequency 2130 Hz downfiled from TMS.arrow_forward
- Predict the chemical shift of the following compounds? find out different types of protons and give their approximate chemical shift values based on their shielding and de shielding.arrow_forward3. Homework Helparrow_forward3. Protons A-E are identified on the spectrum below and correspond to the five unique protons in the molecule shown. (a) Place all the protons on the molecule and categorize them into Ha, Hb, etc. (b) Fill in the table for this molecule (see answer sheet). Br E D C 3 PPM -N B Aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY