
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
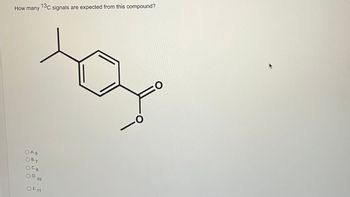
Transcribed Image Text:How many
O A. 5
O B. 7
O C. 8
O D.
13C signals are expected from this compound?
10
O E. 11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12 11 broad singlet 1=1 5. The ¹H NMR spectrum of ibuprofen (the starting material for our reaction) is shown below. Match the peaks of the spectrum with their corresponding protons by filling in the boxes with A-H. A HO 10 D H. E 9 F HHH H₂CH H B C H HG 8 CH3 CH3 H doublet doublet 1=2 1=2 6 5 4 quartet I=1 3 doublet 1=2 doublet 1=3 2 multiplet 1=1 doublet 1=6 0arrow_forwardNMR data Summary NMR solvent: DMSO-d6 'H NMR: Splitting (Multiplicity) Integration Chemical Shift 9.67 singlet 1H singlet doublet 9.17 7.37 6.72 2.00 1H 2H doublet singlet 2H 3H 1"C NMR: Chemical Shift 167.8 153.3 131.1 121.1 115.1 23.8 15N NMR: Chemical Shift 132.3 Instructions: Step 0: Look at the structure of Tylenol. Determine integration, and splitting you are expecting in the 'H NMR. Step 1: Look at the integration and splitting of the signals in the H NMR spectrum - assign the chemical shift of the protons of the methyl group; make a note which signals in the 'H NMR are due aromatic protons, NH and/or OH. Step 2: Look at the 'HSN HSQC spectrum - Use this spectrum to assign the chemical shifts of the NH proton and the OH proton. Step 3: Look at the 'H1SN HMBC spectrum - Use this spectrum to assign the chemical shifts of the aromatic protons in the 'H NMR. Step 4: Look at the 13C NMR spectrum - Make a note which carbon atoms are aromatic and/or alkyl. Assign the chemical shift of…arrow_forwardWhich compound is characterized by showing the peaks M, M + 2 and M + 4 in the region of the molecular ion?arrow_forward
- 2. Which C4H,Br isomers give rise to the two spectra shown below? b. 8 C. 8 7 7 6 5 5 34 4 8 (ppm) 33 32 PPM 4 8 (ppm) 2H t I 2H d 3 ند 3 2H 2H quint sext NH 2 1H m 2 6H d I C 3H 1.0 PPM 0 0arrow_forwardLabel 3 peaks and what functional groups and their wavelength they could possibly correlate with in.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY