
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
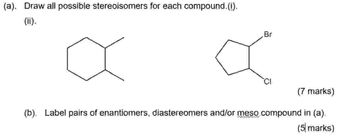
Transcribed Image Text:(a). Draw all possible stereoisomers for each compound. (i).
(ii).
а
Br
т
(7 marks)
(b). Label pairs of enantiomers, diastereomers and/or meso compound in (a).
(5 marks)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- I. Indicate the correct correlation between the following pairs of molecules. Write the appropriate letter in the box. S = Same Compound, E = Enantiomers, D = Diastereomers, and C = Constitutional Isomers. Br and 1. HBr Br 2. and Br and 3. and 4. 2arrow_forwardA)Circle all of the stereo centers in MDMA. B) assign the absolute stereochemistry (R or S) for each stereo centerarrow_forward5. How many stereocenters does this compound have? How many stereoisomers are possible for this compoundarrow_forward
- How to identify constitutional isomers from stereoisomers? Can you provide an example? With molecular formula if it's possible?arrow_forwardOrganic Chemistry, Ch.4, WS #2, Stereochemistry - Review of R&S configuration, Fischer Projections, Enantiomers, Diastereomers, Meso Compounds. 1. Assign R or S configuration to each stereocenter. (a) H₂C H₂C a H- H OH CH₂CH₂ - Br CH, (b) 2. For each Fischer Projection, assign R or S configuration. CH₂ CH₂CH₂ b. H₂N- H COOH + -H CH₂ C-OH (c) HTC OH CH₂OH C. Br- CH₂OH CI CHO CHarrow_forwardOn the molecule below, highlight all R stereocenters in red and all S stereocenters in blue. If it doesn't contain any stereocenters, check the "No Stereocenters" box under the drawing area. H Śarrow_forward
- II. Indicate the correct correlation between the following pairs of molecules. Write the appropriate letter in the box. S = Same Compound, E = Enantiomers, D = Diastereomers, and C = Constitutional Isomers. (? Br CI CI Br 7. and 8. 9. and and Br "Br CI CH3 CI CH3 HBr and CH H- Br and 11. 10. Br Br -H CH,CH3 CH,CH3arrow_forward19) Draw Fischer projections for the following molecules HO H C₂H5 H CI Cillim H J H "!!!!IOH Brill Br OHC COOH HO 20) Draw all possible stereoisomers for 2,3-dihydroxybutane and label any meso compounds.arrow_forward5. Draw the Fisher projection for these compounds. Draw its mirror image then assign R or S configuration. Determine whether they are enantiomers. OH OH HO2C OH OH •CH2OHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY