
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Which of these is the correct rotation for [a] of 0°?
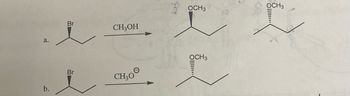
Transcribed Image Text:### Transcription for Educational Website
The image presents two chemical reactions illustrating the substitution of a bromine atom in an organic compound with a methoxy group (OCH₃).
#### Reaction a:
- **Starting Material:**
- A chiral alkyl bromide with the bromine (Br) atom connected to a carbon, shown with a wedge to indicate its spatial orientation.
- **Reagent:**
- Methanol (CH₃OH).
- **Products:**
- Two isomers, where the bromine is substituted by an OCH₃ group.
- Both the methoxy groups (OCH₃) are shown with different spatial orientations:
- One with a wedge indicating that the OCH₃ group is in the same orientation as the original Br.
- The other with a dashed line showing inversion of configuration compared to the original Br.
#### Reaction b:
- **Starting Material:**
- Similar chiral alkyl bromide starting material as in Reaction a.
- **Reagent:**
- Methoxide ion (CH₃O⁻).
- **Products:**
- Similar to Reaction a, it produces two isomers with the methoxy group substituting the bromine, depicted with different configurations of the OCH₃ group:
- One with a wedge (same spatial orientation as original Br).
- Another with a dashed line (inverted orientation compared to original Br).
### Explanation
- Both reactions demonstrate nucleophilic substitution where the bromine is replaced by an -OCH₃ group.
- The use of different reagents (CH₃OH vs. CH₃O⁻) can lead to changes in reaction conditions and possibly reaction mechanisms (like SN1 or SN2).
- The difference in the spatial orientation of the product isomers indicates stereochemistry considerations in such reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Molecule C shown below contains a group called an allene. An allene has the structure of one central carbon being doubly bonded to two other carbons (C=C=C). Please draw out all of the p-orbitals that are involved in the pi-system for molecule C. b) The p-orbital on carbon 1 in molecule C are not in conjugation with the p-orbital on carbon 2 (ie the p- orbitals are not pointing in the same direction and there is no overlap). Why does this happen?arrow_forwardThe structure given is Escitalopram, an active enantiomer of citalopram and works as an anti-depressant. F What is the hybridaization of C10? A sp3 B) sp2 sparrow_forwardFor which of the following molecules are there two unique configurations about the double bond? Explain. (a) (CH3)2C=CHCI; (b) H2C=CHCH,CH3; (c) CIHC=CHBr; (d) HC=CCH=CHCIarrow_forward
- 19. What is the correct classification of the following Newman projection? Br, OH staggered the lowest in energy or anti eclipsed- not the highest in energy eclipsed-highest in energy or total eclipsed staggered -not the lowest in energyarrow_forwardWhich of these structures shows how the pictured molecule will look after it is rotated by 180°, as illustrated Но rotate H. CH, Н, С H,C, OH O: HO, CH, Но но H,C, O: none of these H,C HO Iarrow_forwardWhy does "Zincblende" have a F(-4)3m space group designation? F stands for face centered and the 4-overbar means 90o rotation followed by inversion through the center gives the starting position, but what does the 3m mean?arrow_forward
- For the molecule below, assign configurations (R or S) at C4, C5, and C6. C6 'OH OH C4 Но ОН C5 O C4: R; C5: R; and C6: R C4: S; C5: S; and C6: S C4: S; C5: R; and C6: R C4: R; C5: S; and C6: R C4: R; C5: S; and C6: Sarrow_forwardConsider the following four molecules, count how many sp2 atoms are conjugated how many are non-conjugated. Molecule A has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms. Molecule B has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms. Molecule C has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms. Molecule D has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY