
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
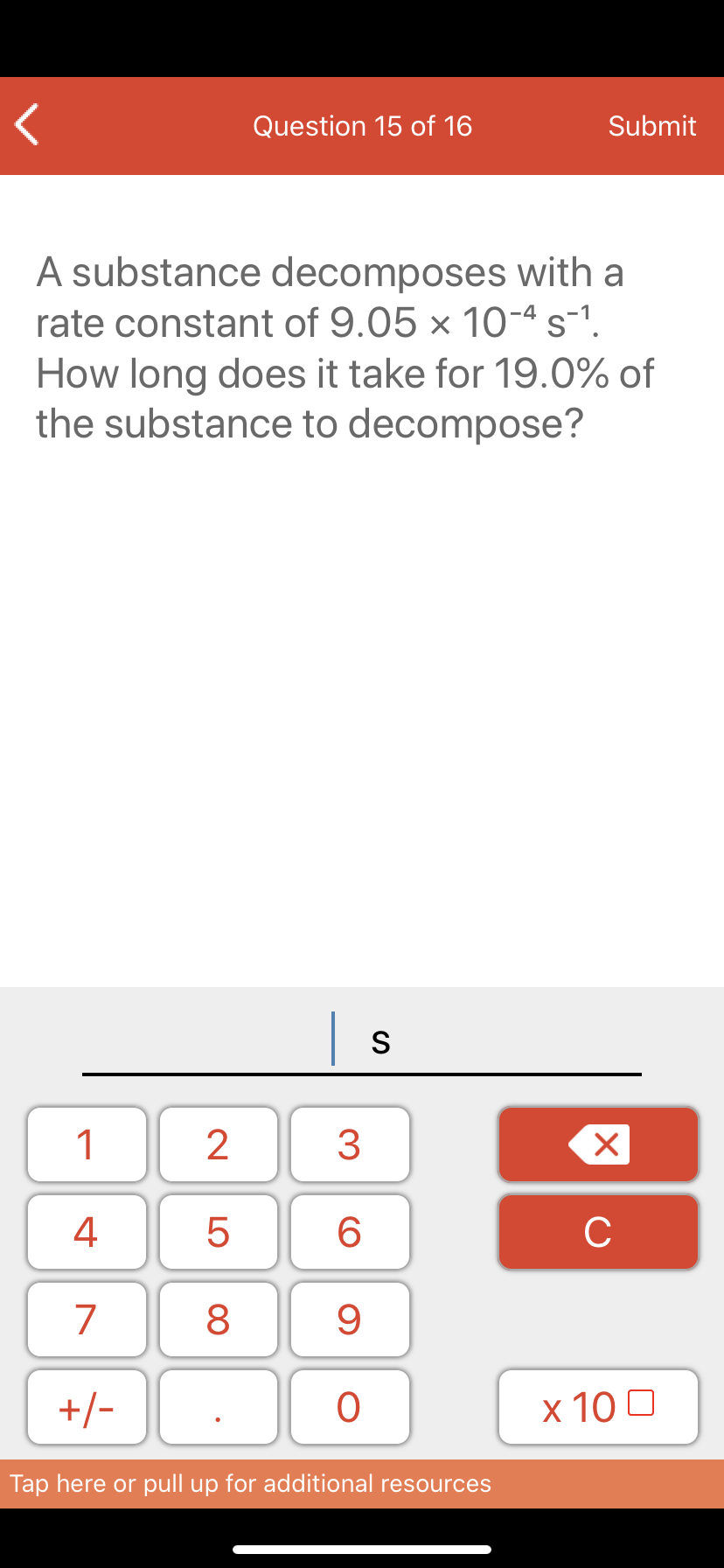
Transcribed Image Text:A substance decomposes with a
rate constant of 9.05 × 10-ª s-1.
How long does it take for 19.0% of
the substance to decompose?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A study of the rate of the reaction represented as X + Y → 2 Z gave the following data: Time (s) 0.0 10.0 20.0 30.0 [A] (M) 0.8324 0.7115 0.6193 0.5463 Determine the average rate of disappearance of X between 20.0 s and 30.0 s, and the rate of appearance of Z between 20.0 and 30.0 s. 1) Average rate of disappearance of X between 20.0 s and 30.0 s = _______ M/s. 2) Average rate of appearance of Z between 20.0 s and 30.0 s = ______ M/s.arrow_forwardThe rate constant at a given temperature for the reaction A →→ 2 B is 0.0145 s¯¹. What is the concentration of B (in molarity) after 1.8 minutes if the initial concentration of A is 2.6 M? Enter your answer without units.arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [N₂] [H₂] initial rate of reaction 1.81M 0.376M 105 M/s 1.81M 0.0561 M 1.19 × 105 M/S 0.236M 0.376M 1.36 × 104 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 2 significant digits. Also be sure your answer has the correct unit symbol. rate = k0.0² 0 8.00 × k = x10 0.0 Xarrow_forward
- What is the concentration of a reactant after 28.5 s if the initial concentration is 0.150 M and the rate constant is 5.4 x 10⁻² s⁻¹?arrow_forwardA substance decomposes with a rate constant of 9.05 × 10⁻⁴ s⁻¹. How long does it take for 21.0% of the substance to decompose?arrow_forwardThe rate constant for a certain reaction is k = 5.10×10−3 s−1 . If the initial reactant concentration was 0.950 M, what will the concentration be after 20.0 minutes?arrow_forward
- 25.) A reaction has a rate constant of k = 0.0870 M-1·s-1. How many seconds will it take for the initial amount of a reactant to decrease from 0.768 M to 0.272 M? Your Answer:arrow_forward5. A certain reaction has a rate law of rate = k[C]. The activation energy for this reaction is 3.0000 x 104 J/mol, and the collision frequency is 2.000 x 10-³. If the desired reaction rate using an initial [C] of 0.0300 M is 1.00 x 10⁹ M/s, what must the reaction temperature be? You must first determine the value of the rate constant k, then use k, EA, and A to determine temperature. Show all work. 6 Find and cito lucing orarrow_forwardWhat is the half life of the following reaction, given the following set of conditions (Hint: observe the units for the rate constant to determine the order of the reaction!): 2 HI (g) → H2 (g) + I2 (g) Initial concentration of HI = 1.00 M k = 1.2 x 10-3 M-1 s-1arrow_forward
- A substance decomposes with a rate constant of 9.05 × 10⁻⁴ s⁻¹. How long does it take for 23.0% of the substance to decompose?arrow_forwardIn a study to determine the rate of the following reaction: 2NO (g) + 0, (g) → 2NO, (g) the concentration of NO was 0.0450 Mat t = 5.0 s and 0.0225 Mat t= 650.0 s. What is the average rate of the reaction during this time period?arrow_forwardIn a study to determine the rate of the following reaction: 2NO(g) +0,(g) 2NO, (g) the concentration of NO was 0.0650 Mat t= 5.0 s and 0.0225 Mat t= 650.0 s. What is the average rate of the reaction during this time period? M/sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY