
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
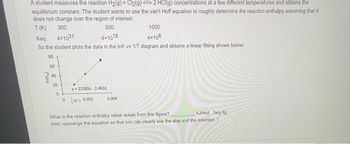
Transcribed Image Text:A student measures the reaction H2(g) + Cl₂(g) <=> 2 HCl(g) concentrations at a few different temperatures and obtains the
equilibrium constant. The student wants to use the van't Hoff equation to roughly determine the reaction enthalpy assuming that it
does not change over the region of interest.
500
T(K) 300
Keq 4x1031
4x1018
So the student plots the data in the InK vs 1/T diagram and obtains a linear fitting shown below:
8 8 8 8
60
40
20
0
0
y-22585x-24632
(0.002
0.004
1000
4x108
What is the reaction enthalpy value obtain from this figure?
kJ/mol 3sig fig.
(hint, rearrange the equation so that you can clearly see the slop and the intercept.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium constant, K., is calculated using molar concentrations. For gaseous reactions another form of the equilibrium constant, Kp, is calculated from partial pressures instead of concentrations. These two equilibrium constants are related by the equation K₂ = K. (RT)An where R = 0.08206 L-atm/(K-mol), T is the absolute temperature, and An is the change in the number of moles of gas (sum moles products - sum moles reactants). For example, consider the reaction N₂(g) + 3H₂(g) = 2NH3(g) for which An=2-(1+3) = -2.arrow_forwardWhich of the following "stresses" applied to the following reaction, initially at equilibrium, would increase the pressure of SO2 in the reaction vessel as the system re-establishes equilibrium conditions?2 CaSO4(s) ⇌ 2 CaO(s) + 2 SO2(g) + O2(g) ΔH° = 1003 kJ * Increase the temperature of the reaction vessel * Add CaO to the reaction mixture * Grind the CaSO4 to increase its surface area * Add a catalyst to the reaction mixture * Remove CaO from the reaction mixturearrow_forwardUsing any data you can find in the ALEKS Data resource, calculate the equilibrium constant k at 25.0 °C for the following reaction. TiC14(g) + 2 H₂O(g) TiO₂ (s) + 4 HC1 (g) Round your answer to 2 significant digits. K = 0 0 Xarrow_forward
- For the reaction 3H2(g)+N2(g)⇌2NH3(g) at 225 ∘C the equilibrium contant is 1.7×10 ^2. If the equilibrium mixture contains 0.19 M H2 and 0.015 M N2, what is the molar concentration of NH3? Express your answer to two significant figures and include the appropriate units.arrow_forwardAt 100 °C, Keg = 1.5E8 for the reaction: CO(g) + Cl2(g) - COCI2(g) Using appropriate approximation, calculate the partial pressure of CO at 100 °C at equilibrium in a chamber that initially contains COCI2 at a pressure of 0.302 bar.arrow_forwardInitial (M) Change (M) Equilibrium (M) Consider the reaction of SO₂ and O₂ described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. 2 SO₂(g) + O₂(g) = 2 SO₂(g) NEXT A 2.00 L reaction vessel was filled 0.0432 mol SO₂ and 0.0296 mol O₂ at 900 K and allowed to react. At equilibrium, the concentration of SO, was found to be 0.0175 M. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. -0.00875 0.0209 0 0.00875 2SO₂(g) Question 20 of 33 2.00 0.0216 0.0432 0.0148 0.0296 0.0041 O₂(g) 0.0175 2 0.0129 -0.0175 0.0061 RESET -0.0350 0.0257 2SO₂(g)arrow_forward
- The equilibrium constant, Kc, for the following reaction is 1.29x10-2 at 600 K. coCI,(g) ? CO(g) + Cl2(g) Calculate the equilibrium concentrations of reactant and products when 0.373 moles of COCI2(g) are introduced into a 1.00 L vessel at 600 K. [COCI2] = [CO] M [Cl2] M ΣΣΣarrow_forwardConsider the hypothetical reaction A(g) 2B(g). A flask is charged with 0.74 atm of pure A. after which it is allowed to reach equilibrium at 0 °C. At equilibrium the partial pressure of A is 0.37 atm Part A What is the total pressure in the flask at equilibrium? Express your answer using two significant figures. VS ΑΣΦ 4 P₁ = Submit Part B What is the value of Kp? Express your answer using two significant figures. K₂= Submit Part C Request Answer |VL ΑΣΦ Submit Request Answer What could we do to maximize the yield of B? → Request Answer [w] ? O Doing the reaction in a larger flask maximizes the yield of B. O Doing the reaction in a smaller flask maximizes the yield of B. atmarrow_forwardFor the equilibrium reaction. 2IBr (g) I2 (g) + Br2 (g) Kc=.0085. If .025 M of IBr is introduced to an empty flask and allowed to reach equilibrium, calculate the final concentrations of all components. Consider the decomposition reaction at 555 K 4POCl3 (g) P4 (g) + 2O2 (g) + 6Cl2 (g) If .450 atm of POCl3 is introduced to an otherwise empty flask and the reaction is allowed to reach equilibrium, the final total pressure is .850 atm. Find Kp and Kc.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY