
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:A student is examining a bacterium under the
microscope. The E. coli bacterial cell has a mass of
m = 1.50 fg (where a femtogram, fg, is 105
and is swimming at a velocity of v =
with an uncertainty in the velocity of 8.00 %. E. coli
bacterial cells are around 1 m (10 m) in
length. The student is supposed to observe the
bacterium and make a drawing. However, the
student, having just learned about the Heisenberg
uncertainty principle in physics class, complains
that she cannot make the drawing. She claims that
the uncertainty of the bacterium's position is greater
than the microscope's viewing field, and the
bacterium is thus impossible to locate.
9.00 pm/s,
9.
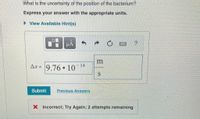
Transcribed Image Text:What is the uncertainty of the position of the bacterium?
Express your answer with the appropriate units.
> View Available Hint(s)
HA
Aæ = 9.76 10
14
Submit
Previous Answers
X Incorrect; Try Again; 2 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- g) A student is examining a bacterium under the microscope. The E. coli bacterial cell has a mass of m = 0.400 fg (where a femtogram, fg, is 10- -15 and is swimming at a velocity of v = 6.00 μm/s, with an uncertainty in the velocity of 1.00 %. E. coli bacterial cells are around 1 μm (10-6 m) in length. The student is supposed to observe the bacterium and make a drawing. However, the student, having just learned about the Heisenberg uncertainty principle in physics class, complains that she cannot make the drawing. She claims that the uncertainty of the bacterium's position is greater than the microscope's viewing field, and the bacterium is thus impossible to locate. Part A What is the uncertainty of the position of the bacterium? Express your answer with the appropriate units. ► View Available Hint(s) ☐☐ Submit 00 xa μA Xh Ax = Value 016 Previous Answers X.10n Units X ?arrow_forwardA student finds that 24.96 g of water at 24.9 C(density=0.9971 g/cm3) is required to completly fill an empty flask. The water is removed and completely dried; granular solid copper weighing 51.24g is then added to the flask. With the copper present in the flask, it was determined that 19.24 g of water was required to fill the remaining space in the flask completly 1.) volume of the empty flask 2.) volume of the copper 3.) density of the copperarrow_forwardA 232 mL cup of whole milk contains about 27 mg of cholesterol. Express the cholesterol concentration of the milk in kilograms per cubic meter (kg/m^3)arrow_forward
- In the year 2013, an estimated amount of 36 billion metrictons (1 metric ton = 1000 kg) of carbon dioxide 1CO22 wasemitted worldwide due to fossil fuel combustion and cementproduction. Express this mass of CO2 in grams without exponentialnotation, using an appropriate metric prefixarrow_forwardYou’re attempting to calculate the density of a titanium alloy. The titanium alloy is in the shape of a cylinder. If the mass of the titanium alloy is 2.56×106g and it has a radius of 3.3meters with a height of 0.0543kilometers what is the density of the alloy in g/m3. [Vcylinder =πr2h][dTitanium=4500kg/m3]arrow_forwardUnit Conversion: 3.05 × 10−1 s to msarrow_forward
- an oral suspension of the antibiotic amoxicillin has a density of 1.72g/cm^3. What is the volume, in cubic inches (in^3), of 0.50Lb of the oral suspension.arrow_forwardthe carat(ct) is a unit of mass used for measuring gemstones and pearls. it is believed that the word comes from Greek for carob seed, because carbo seeds were used to measured jewelry throughout history. in modern times it has been using jewerly measurment, which of the following set up will give the correct converstion for 1.00 troy ounce (t oz) of gold to carat (ct) 24 (g)=1 (dwt) 20(dwt)=1 troy ounce(t oz) 12 (t oz) = 1 troy pound (lb t) 1 (g) =0.0648 (g) 1 carat (ct) =0.200 (g) a) 1.00 t oz=1lb t=12 toz=20dwt=24g=1ct b) 1 toz=1dwt=24g=1gr=20dwt=1lbt=1lb t=1ct c)1.00 t oz=20dwt=24g=1/0.200 ct c)1.00 toz=24g=1gr=1ctarrow_forwardplease see attached imagearrow_forward
- An organic chemist measures the temperature I of a solution in a reaction flask. Here is the result. T = 67. °C Convert I to SI units. Be sure your answer has the correct number of significant digits. ☐K x10 Śarrow_forwardAn atom of helium has a radius He = 31. pm and an average speed in the gas phase at 25°C of 787.m/s. Suppose the speed of a helium atom at 25°C has been measured to within 0.10%. Calculate the smallest possible length of box inside of which the atom could be known to be located with certainty. Write your answer as a multiple of I and round it to 2 significant figures. For example, if the smallest box the atom could be in turns He out to be 42.0 times the radius of an atom of helium, you would enter "42.1" as your answer. esc 0₁ F He Continue 55°F Clear ♫x F1 ! F2 2 F3 # 3 DII F4 x10 Q Search $ X 4 F5 % 5 53₂ F6 L A 6 F7 & © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility P 7 F8 0 F9 DELL prt sc F10 home Submit Assignment F11 end F12 insert E pl + 18 Ar 12:23 5/23/20 deletarrow_forwardA CHM 126 student weighed an empty and clean 50-mL flask. The studernt then used a 10-ml volumetric pipet to transfer deionized water into the flask. Afterwards, she re-weighed the flask with the water. Using her resulting data and the density of water, she determined her measured volume of water in units of mL. Her data is listed below. What was the percent error in her measured milliliters of water? As always, be sure to report your answer with the proper number of significant figures while using proper rounding. mass of empty flask = 62.258 g mass of flask with water = 72.118 g density of water = 0.99821 g/mL Type your answer in the space below without the units.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY