
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
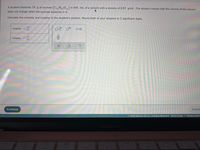
Transcribed Image Text:A student dissolves 14. g of sucrose (C,,H,,0,1) in 450. mL of a solvent with a density of 0.85 g/mL. The student notices that the volume of the solvent
22
does not change when the sucrose dissolves in it.
Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.
molarity = |
x10
molality
%3D
Continue
Submit
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
MacBook Air
20
888
DD
F3
F4
F5
F6
F7
F8
34
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . A solution is A certain liquid X has a normal freezing point of -4.20 °C and a freezing point depression constant K =2.02 °C kg mol prepared by dissolving some benzamide (C₂H NO) in 550. g of X. This solution freezes at -7.7 °C. Calculate the mass of C₂H NO that was dissolved. Round your answer to 2 significant digits. 0 x10 x Sarrow_forwardA chemist must dilute 62.6mL of 2.81M aqueous sodium thiosulfate Na2S2O3 solution until the concentration falls to 2.00M. He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Be sure your answer has the correct number of significant digits.arrow_forwardHow many grams of water must be used to prepare a 17.0 percent by mass (%m/m) sodium hydroxide (NaOH) solution from 7.80 g NaOH? Report your result in decimal notation and to the proper number of significant figures. All numbers are measured. a) 83.0 g waterb) 98.8 g waterc) 38.1 g waterd) 218 g watere) 92.2 g waterarrow_forward
- How many grams of water must be used to prepare a 2.50 percent by mass (%m/m) sodium hydroxide (NaOH) solution from 12.7 g NaOH? Report your result in decimal notation and to the proper number of significant figures. All numbers are measured. a) 2.50 g water b) 5.08 g water c) 12.7 g water d) 495 g water e) 508 g waterarrow_forwardYour know-it-all classmate just explained to the class that NaCl is a gas because the line on the graph is basically flat. Is your classmate correct? Explain your reasoning. 100 NaNO, 80 06 70 60 CaCl 50 Pb(NO)2 40 NaCI KCI 30 20 KCIO, 10 Ce,(SO 0. 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Solubility (g of salt in 100 g H,O) CONSarrow_forwardAn aliquot of 49.47 mL of methanol is mixed with water such that the total volume of the solution is 170.00 mL. Determine the concentration of methanol in percent by volume (% v/v). Report answer to the correct number of significant figures.arrow_forward
- Calculate the volume in liters of a 0.163M potassium dichromate solution that contains 100. g of potassium dichromate (K,Cr,0,). Be sure your answer has the correct number of significant digits. L x10arrow_forwardWhat is the normality of a solution made by dissolving 10.00 g of hydrogen phosphate, H3PO4 in water to a volume of 150 mL? Note: Please present complete solution. Express your final answers up to two (2) decimal places.arrow_forwardObserve the graph below. According to the chart shown above, if the saturated solution of glucose at 80°C was suddenly cooled to 40°C, what would happen? Solubility of Glucose in Water 600 S00 400 300 200 100 20 40 80 100 Temperature (Celsius) An additional 300 grams of glucose could dissolve. O The solution would spontaneously cool to 200°C. O The solution becomes supersaturated. O Nothing special would happen; the increased temperature just made the dissolving process quicker. Dissolved Mass (grams)arrow_forward
- The solubility in water of ionic compound X is measured and found to be 77.3 g at 25. °C. Calculate the volume of a saturated solution of X in L water that would contain 50.0 g of X at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits. x10 0.0 X μarrow_forwardMay you answer and explain this please?arrow_forwardA chemist prepares a solution of barium chlorate by measuring out 28.6 implying of barium chlorate into 100mL volumetric flask filling the flask to the mark with water. Calculate the concentration in mol/ L of the chemist's barium chlorate solution. Round your answer to 3 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY