
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
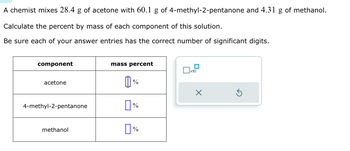
Transcribed Image Text:A chemist mixes 28.4 g of acetone with 60.1 g of 4-methyl-2-pentanone and 4.31 g of methanol.
Calculate the percent by mass of each component of this solution.
Be sure each of your answer entries has the correct number of significant digits.
component
acetone
4-methyl-2-pentanone
methanol
mass percent
Ï%
0
п%
%
☐
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 63 gallons of a 26% acid solution are mixed with 44 gallons of a 80% acid solution. What is the acid concentration of the resulting mixture? Round to the nearest percent.arrow_forwardIn this task, you are to prepare 3 × 100 mL solutions of acetic acid at 3 different concentrations. You are provided with a stock solution at 2 % and will need to perform 3 dilutions. Complete the table below to calculate how much of the stock solution and how much water is required to prepare these solutions. For this calculation the following equation may be useful: C₁ V₁ = C₂ V2 Where C₁ is the initial concentration of the stock solution, V₁ is the amount of stock solution you need to add, C2 is the final concentration that you desire and, V2 is the final volume that you desire. All the values for this equation have been provided, aside from V₁ which you will need to solve the equation to determine. + Solution 2 Acetic acid at 0.5 % Solution 3-Acetic acid at 0.2% Solution 4 - Acetic acid at 0.1% Amount of stock solution to add A student uses the 1% acetic acid stock solution to make a new dilution for an experiment. The student takes 32 mL of the stock solution and makes a dilution…arrow_forwardA certain liquid X has a normal freezing point of -6.20 °C and a freezing point depression constant K, = 5.32 °C kg-mol. Calculate the freezing point of a solution made of 3.7g of sodium chloride (NaCl) dissolved in 100. g of X. Round your answer to 3 significant digits. 0°C X 0 Aarrow_forward
- A certain liquid X has a normal freezing point of -3.10 °C and a freezing point depression constant K₁=5.02 °C-kg-mol. A solution is prepared by dissolving some sodium chloride (NaCl) in 300. g of X. This solution freezes at -4.1 °C. Calculate the mass of NaCl that was dissolved. Round your answer to 2 significant digits. 1 g 1 x10arrow_forward4. Hexachlorophene is used as a disinfectant in germicidal soaps. What mass of hexachlorophene ( M = 406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C? K = 3.63°C/m, boiling point of pure chloroform = 61.70°C %3Darrow_forwardA chemist mixes 59.3 g of acetone with 97.8 g of 2-ethyltoluene and 47.1 g of isopropenylbenzene. Calculate the percent by mass of each component of this solution. Be sure each of your answer entries has the correct number of significant digits. component mass percent x10 % acetone 2-ethyltoluene isopropenylbenzene 0%arrow_forward
- A chemistry student needs 85.0 g of isopropenylbenzene for an experiment. He has available 0.20 kg of a 14.8% w/w solution of isopropenylbenzene in carbon tetrachloride. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to 3 significant digits. No x10 solutionarrow_forwardAn impure sample contains 0.78 g of impurities and 4.95 g of benzoic acid. The sample is dissolved in 100 mL of water and heated. The solution is then cooled. How many grams of the impurity will crystallize when the solution has cooled? The solubilities of the impurity and benzoic acid in cold and hot water are given below. Compound Cold Water solubility Hot Water Solubility Benzoic acid 0.42 g per 100 mL of water 5.79 g per 100 mL of water Impurity 0.61 g per 100 mL of water 4.62 g per 100 mL of waterarrow_forwardyou are given 15ug/ml atrazine to prepare a 3 ppb solution a. use te terms working and stock to describe the 2 solutions b. describe the 15 ug/ml atrazine solution in X terms c. what concentration would a 10X stock of atrazine be if you will use it at 3 ppb. You may express your answer in units of ppb.arrow_forward
- In the laboratory you are asked to make a 0.170 m ammonium acetate solution using 455 grams of water. How many grams of ammonium acetate should you add? grams.arrow_forwardA 7 mass % aqueous solution of ethylene glycol (HOCH2CH2OH) has a density of 1.37 g/mL. Calculate the molarity of the solution.arrow_forwardData Table Mass of Zn HCI Volume Tinitial Tfinal 0.55 g 100.0 mL 20.0 °C 23.1°C The density of the 6M HCI is 1.07 g/mL. Which of the following shows how to convert the volume of the solution used into grams of solution? O a. 100.0 mL x 1mL 1.07 g Ob. 1.07 g 100.0 mL 1.07 g 1ml OC. 100.0 ml x The amount of heat absorbed by the solution can be found using the equation q = m x SH x AT. It can also be written as qsolution = msolution X SHsolution X ATsolution: We can assume that the specific heat of the solution is the same as the specific heat of water which is 1.00 cal/(g °C). Data Table Mass of Zn HCI Volume T;initial Tfinal 0.55 g 100.0 mL 20.0 °C 23.1°c Which of the following shows the correct calculation of gsolution? cal "x 3.1°C g°C O a. 100.0 mL x 1.00 Ob. cal 107 grams x 1.00 g °C х 3.1°C 0.55 grams x 1.00 cal х 3.1°C g °Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY