
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
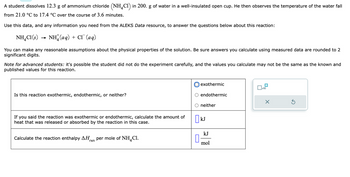
Transcribed Image Text:A student dissolves 12.3 g of ammonium chloride (NH4Cl) in 200. g of water in a well-insulated open cup. He then observes the temperature of the water fall
from 21.0 °C to 17.4 °C over the course of 3.6 minutes.
Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction:
NH4Cl (s)
NH(aq) + CT (aq)
You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 2
significant digits.
Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and
published values for this reaction.
Is this reaction exothermic, endothermic, or neither?
If you said the reaction was exothermic or endothermic, calculate the amount of
heat that was released or absorbed by the reaction in this case.
Calculate the reaction enthalpy ΔΗ per mole of NH Cl.
rxn
n
exothermic
endothermic
neither
kJ
mol
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Defining system and surrounding for the given dissolution process
VIEW Step 2: Finding out whether reaction is exothermic or endothermic
VIEW Step 3: Calculation for heat absorbed
VIEW Step 4: Calculation for moles of Ammonium chloride
VIEW Step 5: Calculation for enthalpy of reaction per mole
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student dissolves 13.4 g of sodium chloride (NaCl)in 250. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 20.0 °C to 18.9 °C over the course of 6 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaCl (s) Na+ (aq) + CI (aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy ΔΗ per mole of NaCl.…arrow_forwardA student mixes 67.0 mL of a 2.01 M sodium hydroxide solution with 22.4 mL of 6.45 M hydrochloric acid. The temperature of the mixture rises 17.2 ° C. The density of the resulting solution is 1.00 g mL and has a specific heat capacity of 4.184 J g · ° C . The heat capacity of the calorimeter is 16.97 J ° C . Part 1: (a) Identify the limiting reagent for the reaction. Part 2: (b) Calculate the heat of reaction (in J). qrxn = × 10 JEnter your answer in scientific notation. Part 3 out of 3 (c) Find the enthalpy of neutralization (in kJ/mol). ΔHneutralization = ____ kj/molarrow_forwardA student dissolves 10.2 g of lithium chloride (LİCI)in 250. g of water in a well-insulated open cup. She then observes the temperature of the water rise from 20.0 °C to 27.1 °C over the course of 7.5 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: LICI(s) Li* (aq) + CI (aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 2 significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. exothermic Is this reaction exothermic, endothermic, or neither? endothermic neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. |kJ kJ Calculate the reaction enthalpy…arrow_forward
- A student dissolves 14.0 g of sodium hydroxide (NAOH) in 250. g of water in a well-insulated open cup. He then observes the temperature of the water rise from 23.0 °C to 36.7 °C over the course of 8.4 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaOH(s) - Na (aq) + OH (ag) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic Oneither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the…arrow_forwardA student dissolves 10.8 g of sodium chloride (NaCl)in 200. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 20.0 °C to 18.9 °C over the course of 6.4 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaCl(s) Na+ (aq) + C₁ (aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 2 significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy ΔΗ. per mole of NaCl. rxn O 1 exothermic…arrow_forwardA 0.454-g sample of xanthone (C13H8O2) is burned in a bomb calorimeter and the temperature increases from 24.00 °C to 26.70 °C. The calorimeter contains 1.02×103 g of water and the bomb has a heat capacity of 771 J/°C. Based on this experiment, calculate ΔE for the combustion reaction per mole of xanthone burned (kJ/mol). C13H8(s)+14O2(g)=13O2(g)+4H20(l)arrow_forward
- A student dissolves 12.8 g of ammonium nitrate (NH4NO₂) in 200. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 22,0 °C to 16.5 °C over the course of 5.6 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NH NO3(s)→ NH(aq) + NO3(aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 2 significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy AH per mole of NH NO rxn exothermic…arrow_forwardA student dissolves 14.0 g of ammonium nitrate (NH4NO3) in 200. g of water in a well-insulated open cup. He then observes the temperature of the water fall from 22.0 °C to 16.6 °C over the course of 5.5 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NH4NO₂ (s) NH(aq) + NO3(aq) - You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy AHxn per mole…arrow_forwardA student dissolves 11.9 g of sodium chloride (NaCl)in 250. g of water in a well-insulated open cup. He then observes the temperature of the water fall from 23.0 °C to 22.0 °C over the course of 6.9 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: + NaCl (s) - Na" (aq) + Ci¯ (aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. exothermic x10 Is this reaction exothermic, endothermic, or neither? endothermic neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. kJ kJ…arrow_forward
- Classify the following reactions as endothermic or exothermic.arrow_forwardA student dissolves 13.2 g of sodium hydroxide (NaOH)in 300. g of water in a well-insulated open cup. She then observes the temperature of the water rise from 21.0 °C to 33.4 °C over the course of 5.3 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: + NaOH (s) — Na" (ад) + ОН (ад) olo You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 3 significant digits. Ar Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. exothermic x10 Is this reaction exothermic, endothermic, or neither? endothermic neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. kJ kJ Calculate the…arrow_forwardHelp with the following questionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY